Published Jack Cochran on September 19, 2018
The aromatic-olefins, like styrene and its analogs, have distinctly different VUV absorbance spectra versus their aromatic-aliphatic counterparts as shown in a recent post. But that’s not where the story ends. Aromatic-alkynes, e.g., phenylacetylene, can be present as impurities in commercial styrene used for making polymers and lead to poisoning of catalysts that are used for their removal. GC-VUV can play a role in accurate quantification for aromatic-alkynes, even when they coelute with other compounds, based on their characteristic spectra.
Figures 1-3 contrast phenylacetylene, styrene, and ethylbenzene VUV spectra. While these compounds do not chromatographically coelute with each other, it’s easy to see their spectra are unique, which means they can be determined in the presence of other coeluting compounds that may be much higher in concentration.
The diyne (a word that would make a great crossword puzzle answer!), 1,3-diethynylbenzene, has a VUV spectrum that is more remarkable, with substantial absorbance in the 200-240 nm range (Figure 4). More absorbance features in the higher wavelength range lead to better selectivity and better sensitivity, desirable advantages afforded by GC-VUV for complex sample analysis.
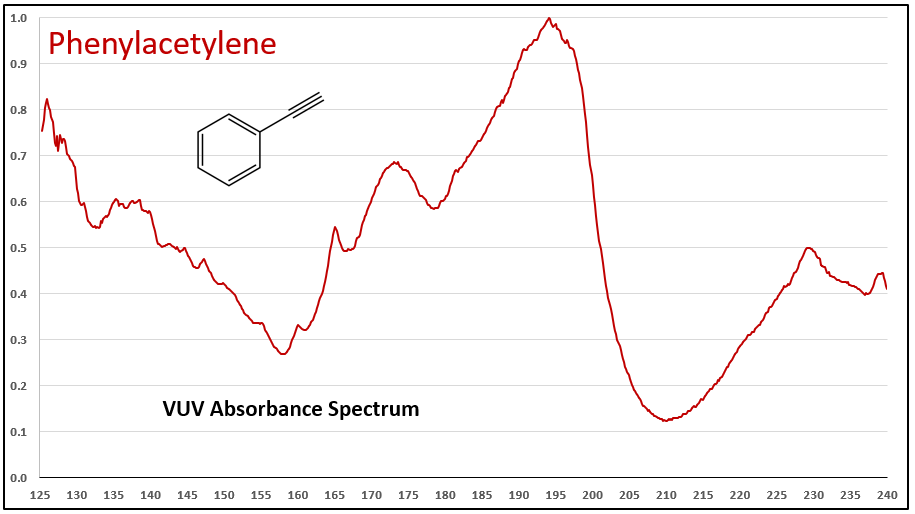
Figure 1
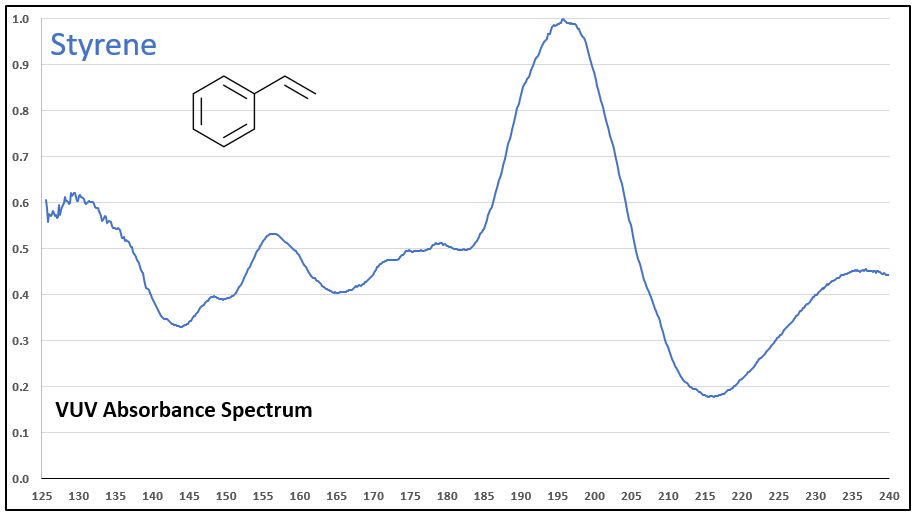
Figure 2
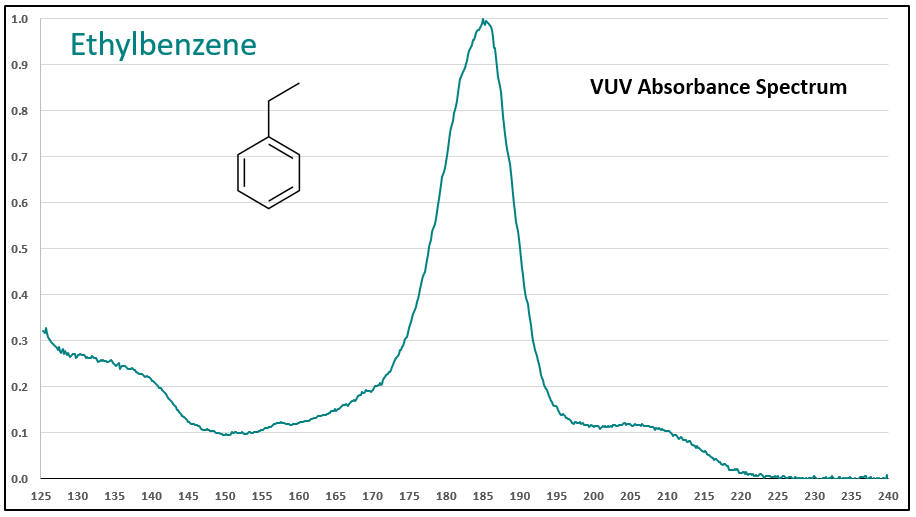
Figures 1-3. VUV absorbance spectra for phenylacetylene and two analog compounds, styrene and ethylbenzene. The aromatic-alkyne (phenylacetylene) and aromatic-alkene (styrene) show increasing absorbance in the 210-240 nm range, where the aromatic-alkane (ethylbenzene) does not. All of the spectra are unique.
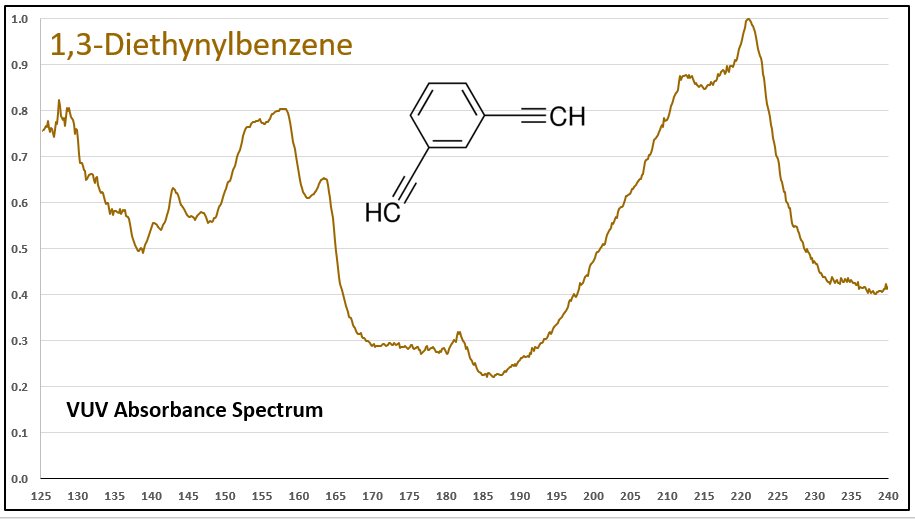
Figure 4. VUV absorbance spectrum for the diyne, 1,3-diethynylbenzene. Note the absorbance in the higher wavelength range, which offers excellent selectivity and sensitivity when analyzing complex samples by GC where coelutions can occur.








Leave a Reply