Published Jack Cochran on June 18, 2018
The previous post on analysis of triethylene glycol showed an analyte list that included water and select hydrocarbons, e.g., benzene. Those of you who know I’m picky about my chromatography may have noticed that I tolerated some peak tailing on water, which was necessitated by the lower GC oven temperature start of 100°C to avoid a coelution with the diluent ethanol used for the work. But there is something I know that you don’t know; well, I guess you’ll know it now since that is what this post is all about. There is a second compound eluting under the water peak, a small amount of formaldehyde that is a contaminant in the triethylene glycol. As seen in Figure 1, we can easily deconvolute these two compounds because they have distinct VUV absorbance spectra. AND, both compounds, water and formaldehyde, are not detected when using a flame ionization detector! That’s what I mean about the exponential power of GC-VUV: determining things you normally can’t see with other GC detectors and identifying and quantifying them in an unbiased fashion when they coelute.
GC-VUV for the Simultaneous Determination of Water and Volatile Petroleum Hydrocarbons in Triethylene Glycol Used for Natural Gas Dehydration
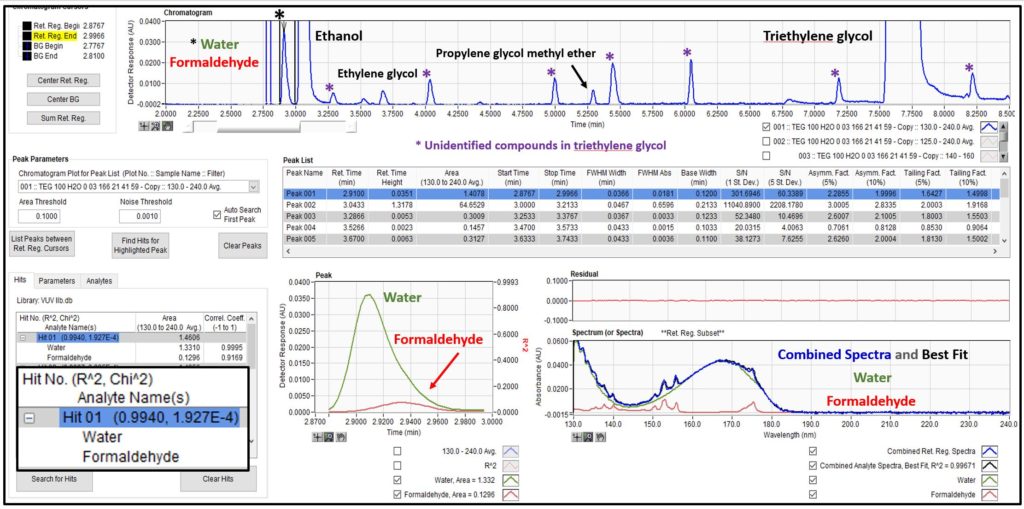
Figure 1. Water and formaldehyde are detected in triethylene glycol using GC-VUV. Their unique absorbance spectra allow deconvolution when they chromatographically coelute.








Leave a Reply