Published Dan Wispinski on May 11, 2020
As an expert in the fuels analysis industry for over 30 years, I have compiled a three-part series on the history of DHA, the current technique and future technologies
A Historical Perspective
In the 1990s, gasoline regulations were changing rapidly. Lead antiknock compounds were banned. Typical gasoline benzene levels were as high as 7.5% vol. Gasoline compositional impacts on the environment and human health were under scrutiny. Regulators were imposing maximum levels for sulfur, benzene, organo-metallic additives and minimum levels of deposit control additives to improve air quality. Oxygenates also began to be added to gasoline for their environmental benefits. New test methods were needed to measure and control these parameters. The EPA cited ASTM test methods D1319 (olefins), D3606 (benzene), D5599 (oxygenates) and D5769 (aromatics). Europe chose a complex multidimensional GC technique equivalent to ASTM D6839. All these techniques have advantages and disadvantages (Figure 2).
Canada and Japan chose a different path – detailed hydrocarbon analysis (DHA) (Figure 1). JIS K 2536-2 was developed in Japan. CAN/CGSB 3.0 No. 14.3 was developed in Canada, which are like ASTM D6729 and D6730. In Canada, I was personally involved in DHA method development and the process in choosing referee gasoline methods. DHA’s advantages were clear:
- Extremely good precision, especially for benzene
- Single method versus multiple techniques
- Robust – a single column split/splitless FID GC is about as simple as it gets
- Stability – columns and retention times/indices can last for several years
- Calibration free –- hydrocarbon theoretical mass response factors and normalization calculations
DHA is indispensable for refineries to optimize processes, value streams and to certify products. DHA is also very versatile, going far beyond routine regulated parameters and refinery applications. The technique has the ability to speciate and quantitate over 400 gasoline boiling range components. By characterizing hundreds of gasoline samples and using equation of state modeling, Canada was able to quantitate fugitive hydrocarbon emissions across the country to justify vapor recovery mitigation strategies. Health Canada used the data to equate the reduction of benzene emissions to number of lives saved. DHA was used when NTSB investigators wanted to know the hydrocarbon composition in the vapor phase of the fuel tank in the ill-fated TWA 800 flight. Underground fuel storage tank leaks are a huge environmental problem. DHA is often used to identify, trace and fingerprint hydrocarbon plumes in ground water. Automotive manufacturers use DHA results to model tailpipe emissions and assist engine design.
Esoteric applications aside, DHA is routinely used to calculate and correlate to other chemical and physical properties such boiling point distribution, bromine number, octane, heat of combustion, particulate matter index and vapor pressure. No other afore mentioned regulatory method provides the wealth of information than that of DHA.
DHA Today
It has been 30 plus years since inception and the DHA technique has not changed; the hardware and software are essentially the same. If a column has not been changed in five years, the retention time of benzene is still the same. The advantages of DHA are evident but there are drawbacks:
- Long run times
- Coelutions
- Misidentifications
- Oxygenate peak tailing
- Chromatogram integration
- Always some amount of “unknowns”
- Requires a highly skilled operator
I’ll be back next month to talk about new technologies and the future of DHA with a new technique called Verified Hydrocarbon Analysis™ from VUV Analytics.
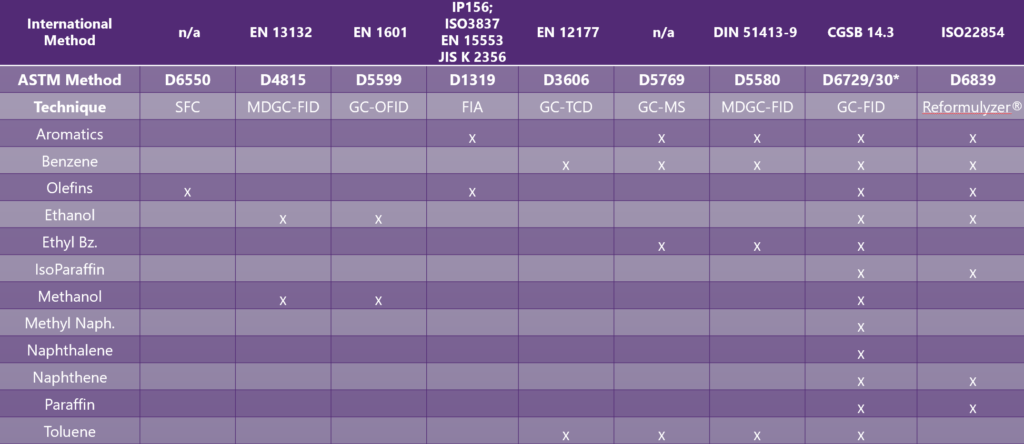
Figure 1. A comparison of global regulated methods for gasoline certification and what they measure.
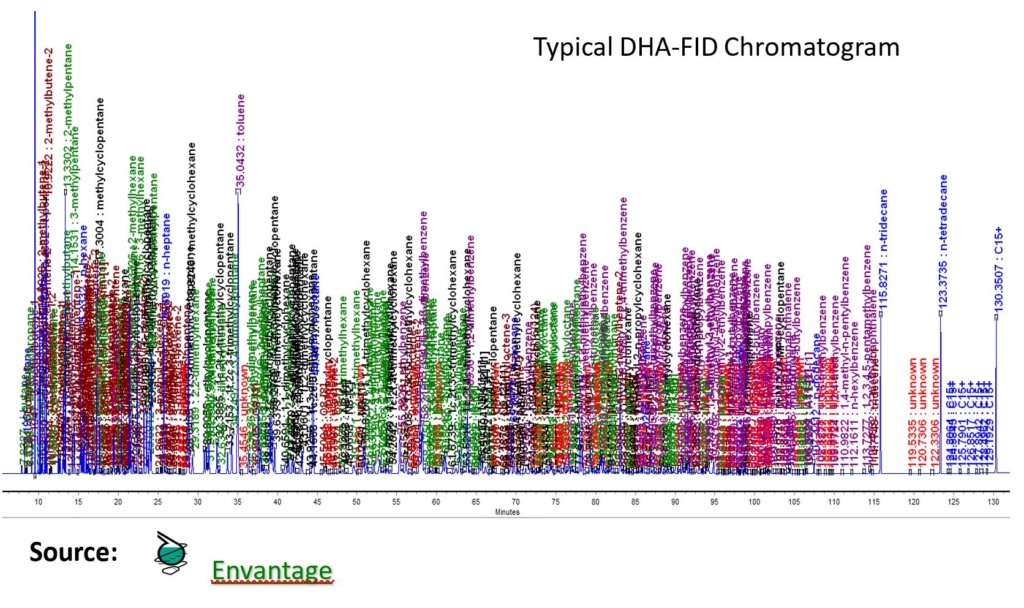
A typical Chromatogram from DHA with FID showing more than 230 compounds and coleutions.









Interesting
Very well Explained. One of the additional challenges with DHA are
1. Different databases for different types of samples.
2. Time for adjustments of database after long analysis time
3. Difficulty in identifying component(Group) after C8, which is easily overcame by VUV especially for group identification.