Published Ryan Schonert on August 15, 2018
Isomeric Distinction in Drugs of Abuse
When performing an analysis on a sample, it’s important to recognize the limitations of your analysis. Before any work is done, you should consider each possible analysis technique and ask, “Is my sample amenable to this technique?” and, “Will this technique give me useful information?” If the answer to either of these questions is no, other options should be considered.
In the realm of GC detectors, each has its advantages and disadvantages. A flame ionization detector (FID) can easily detect saturated hydrocarbons, but it struggles with highly oxygenated and halogenated compounds. An electron capture detector (ECD) has great sensitivity with analytes such as halogenated compounds, but that sensitivity decreases as electronegativity decreases. On the other hand, a mass spectrometer (MS) is a “universal” detector, and it can both detect compounds and identify those compounds through spectral library searching. Given its popularity, I once thought MS to be infallible, but is MS really the king of GC detectors?
As I mentioned in my previous post, traditional GC detectors, including MS, are limited in their run times due to the need for chromatographic separation. Unfortunately for MS, it has another weakness; it has a hard time distinguishing structural isomers. Many labs have a need for structural isomeric distinction, including forensics labs. For example, in the realm of drug abuse, structural isomers of synthetic drugs are quite common. Consider the following pairs of isomers; one is a pair of synthetic cathinones (Figures 1 and 2) and the other is a pair of synthetic opioids (Figures 3 and 4).
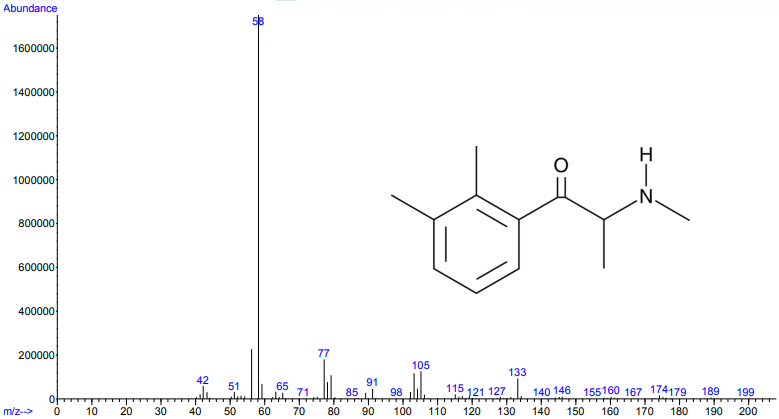
Figure 1.
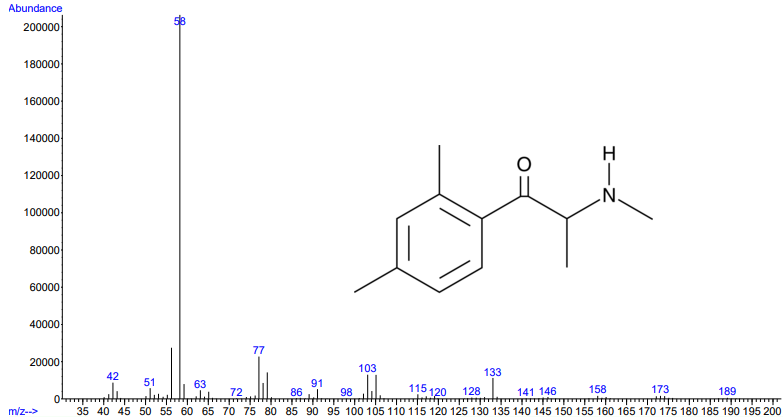
Figures 1 and 2. Electron ionization mass spectra of 2,3-dimethylmethcathinone (top) and 2,4-dimethylmethcathinone (bottom). Retrieved from Cayman Chemical.
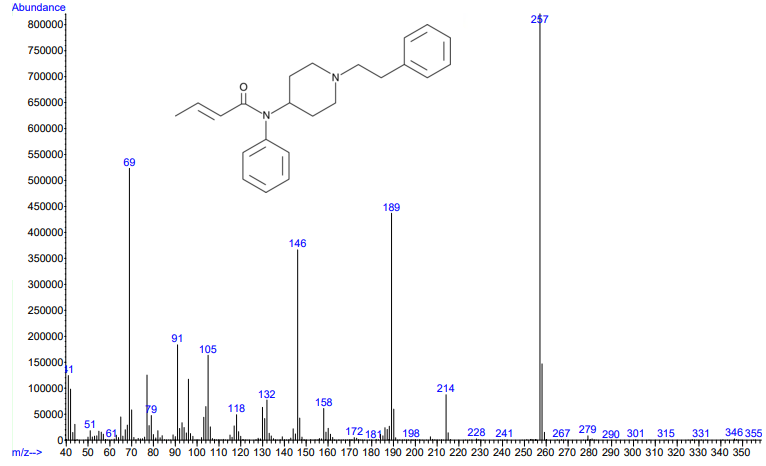
Figure 3.
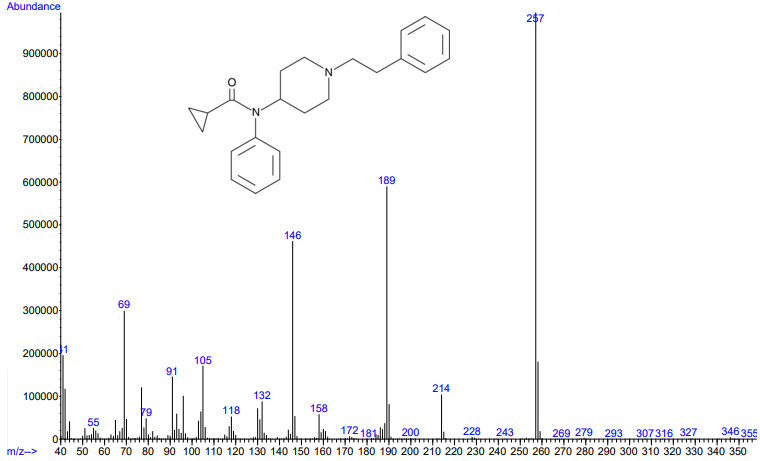
Figures 3 and 4. Electron ionization mass spectra of crotonyl fentanyl (top) and cyclopropyl fentanyl (bottom). Retrieved from Cayman Chemical.
As you can see from the figures, in these cases mass spectra in the same compound class share the same m/z ions. What if you were an expert analyst working a case where the difference between two structurally similar isomers was important to the case’s outcome? As a former student of forensic science, I imagine that it would be nearly impossible to convince a jury that I could tell these compounds apart; the mass spectra are simply too similar. Instead, we can look at the VUV absorbance spectra, shown in Figures 5 and 6. Suddenly, the daunting task of distinguishing nearly identical compounds becomes all too easy.
In this scenario, explaining the differences between these pairs of compounds to a jury is significantly easier. For example, regarding the fentanyl isomers, the crotonyl fentanyl isomer has a pronounced absorption shoulder around 210 nm, while the cyclopropyl fentanyl isomer has a larger absorption in the 170 nm – 190 nm region. These differences are clearly visible even to those without an analytical eye.
When it comes to isomeric distinction, VUV is the clear winner. Once again, the limitations of traditional chromatography and traditional detectors can be overcome with VUV.
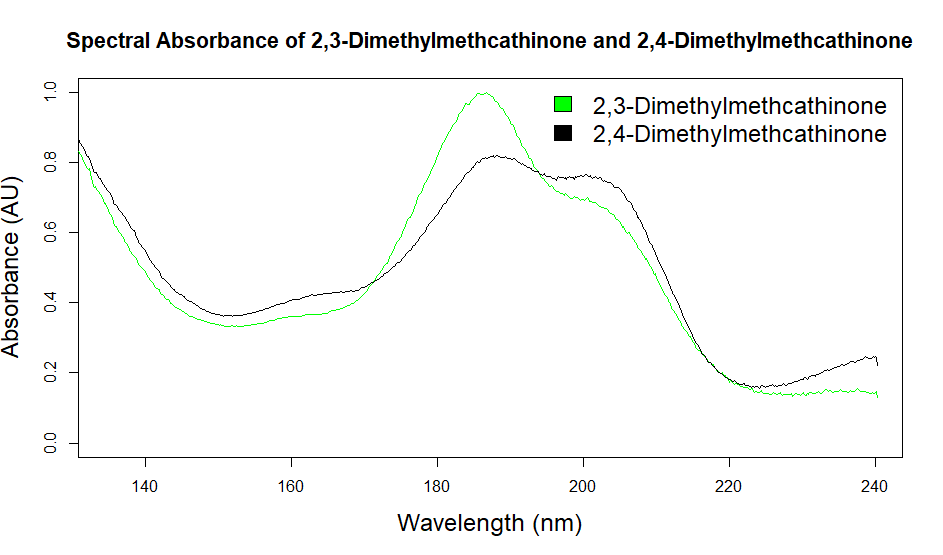
Figure 5. Overlaid VUV absorbance spectra of 2,3-dimethylcathinone and 2,4-dimethylmethcathinone. These isomeric compounds have unique spectra, allowing them to be easily distinguished from each other.
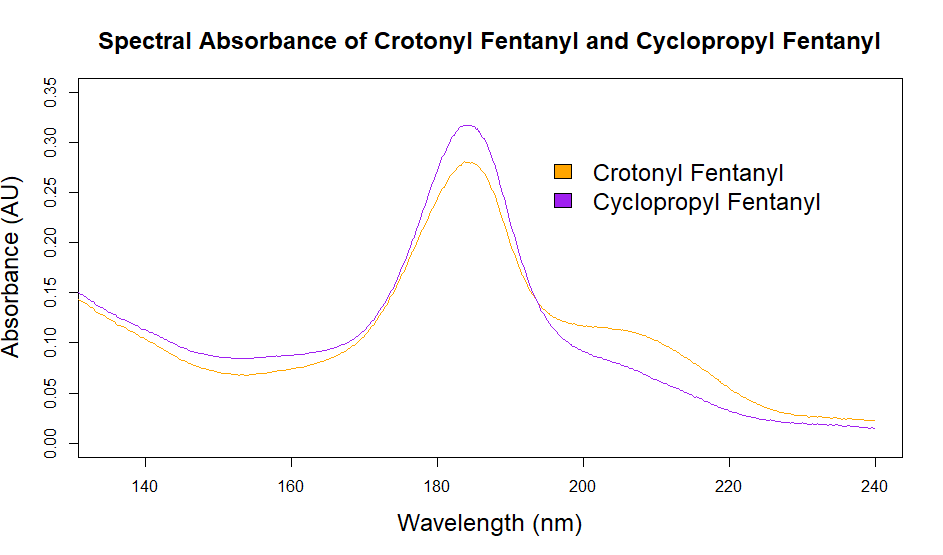
Figure 6. Overlaid VUV absorbance spectra of crotonyl fentanyl and cyclopropyl fentanyl. The VUV spectra are unique, unlike the mass spectra, which are almost identical.










Leave a Reply