Published Ryan Schonert on July 20, 2020
As many of you know, VUV Analytics has its roots in fuels analysis. Although the original VGA-100 detector had many theoretical uses at its inception, methods of fuels analysis such as ASTM D8071-19 were among the first major commercial applications, and we’re continually working to further improve these methods today. However, the VGA-100 and VGA-101 are universal detectors, so they can be applied to several industries. Because of my background, I love exploring the forensics applications of VUV spectroscopy. Let’s take a look at a lesser-known application: fire debris analysis.
Even Fires Leave Evidence Behind
Arson is a centuries-old crime, but for most of human history, eyewitness accounts were the only tool we had to investigate the origins of suspicious fires. Although destructive, fires still leave behind useful information, and we can take advantage of this to reconstruct crimes of arson. Fire debris is partially burned material from the scene of a fire that may contain traces of an ignitable liquid or accelerant; for example, if an arsonist used gasoline at the scene, traces of that gasoline may survive and remain trapped in debris at the scene.
These traces can be analyzed to profile that gasoline, which may be useful in the investigation. Do you see where I’m going with this? VUV might just be the perfect marriage of forensics and fuels analysis by VUV! VUV is a powerful tool in fuels analysis, and by bringing that power to fire debris analysis, there are a ton of possibilities! Fire debris analysis is often done by GC-MS so that chromatograms can be matched for their general patterns and MS can be used to identify prominent peaks. This can work, but I hypothesized that VUV could offer a lot more information in its use of spectral deconvolution and identification.
Analysis of Residual Gasoline
To test this out, I performed some small-scale experiments using various gasolines and diesels, as well as paint thinner, charcoal lighter fluid, and mineral spirits – don’t worry, no crimes were committed during this experiment. I used methylene chloride to do a simple solvent extraction of the fire debris and ran the samples by GC-VUV, using VUV Analyze™ to handle the automated data analysis. Here’s what I found.


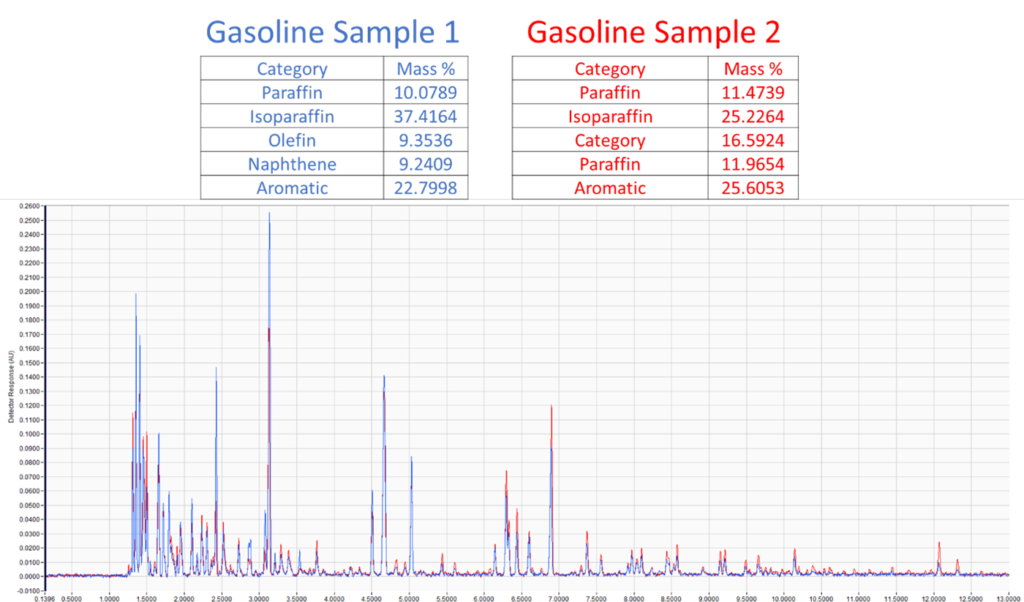
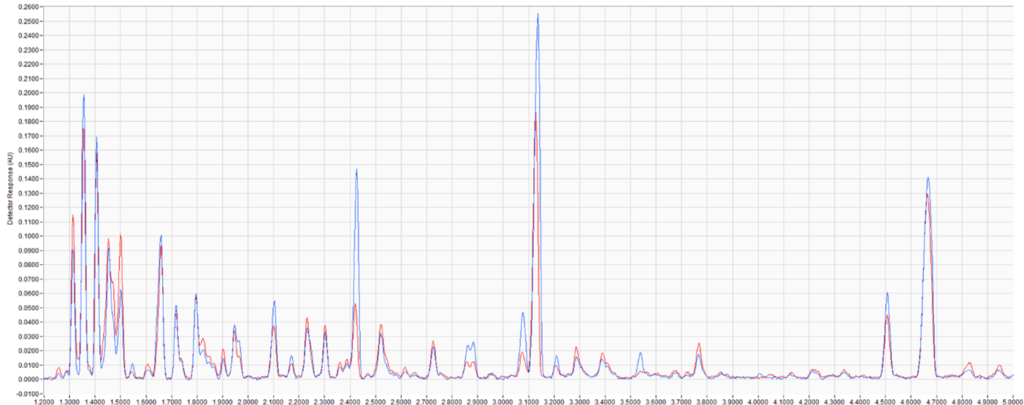
Figure 1. Comparison of paraffin, isoparaffin, olefin, naphthene, and aromatic (PIONA) content of two gasoline samples extracted from simulated fire debris (top). Chromatographic overlay of the two extracts (middle, bottom). Notice that the samples have many of the same peaks, but they are easily distinguishable by PIONA analysis.
In this example, two of the gasoline samples have many of the same peaks, which could make it more difficult to distinguish them by chromatographic pattern matching alone. Additionally, because the samples mostly share the same compounds, finding individual compounds unique to each sample would also be difficult. However, by taking advantage of VUV’s quantitative power, we’re able to measure the paraffin, isoparaffin, olefin, naphthene, and aromatic (PIONA) content of each sample. Looking at the results, an analyst could easily distinguish these two samples!
Analysis of Residual Diesel Fuel
Now, you might be wondering: aren’t there a lot of light compounds that would normally have been evaporated off of the sample? It’s true, those chromatograms are showing some light compounds in the C5-C6 range that would probably be driven off the sample in most cases. However, even if we were use samples that were composed of heavier hydrocarbons, we would still be able to distinguish them. To demonstrate this, we can take a look at the analysis the diesel samples.

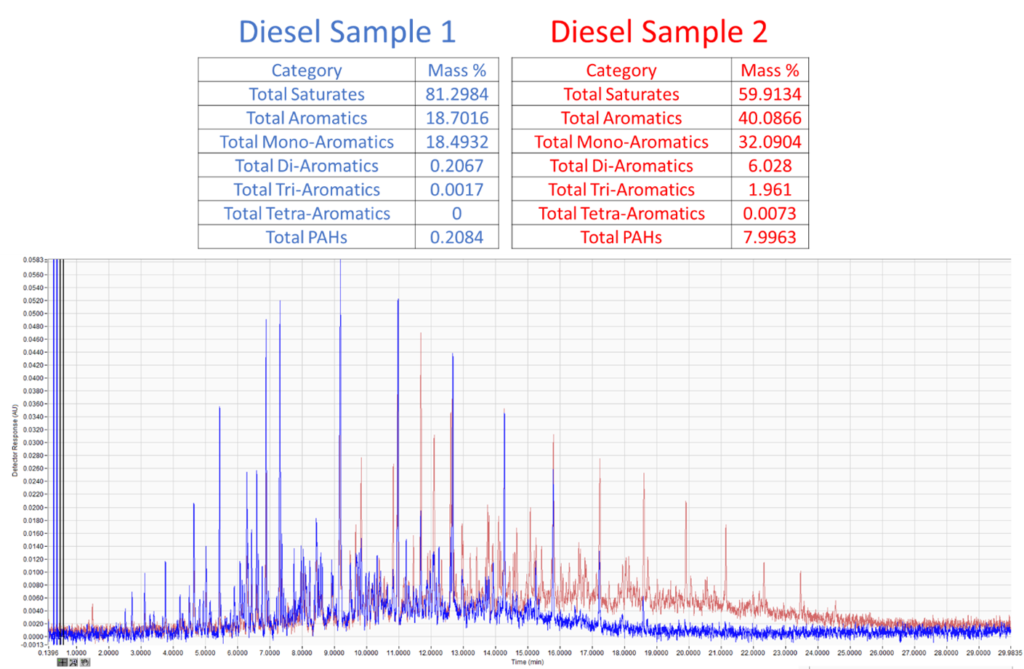

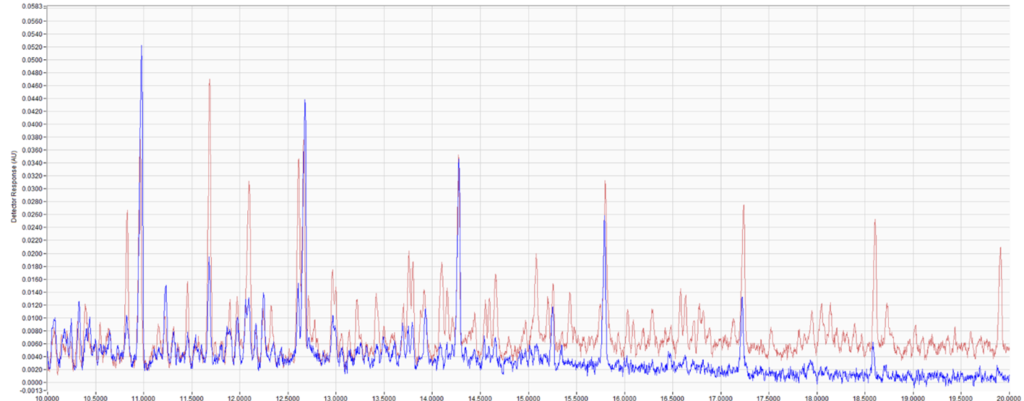
Figure 2. Comparison of saturate and aromatic content of two diesel samples extracted from simulated fire debris (top). Aromatics can be broken up into mono-aromatics, di-aromatics, tri-aromatics, tetra-aromatics, and PAHs. Chromatographic overlay of the two extracts (middle, bottom). Although these samples are visually distinguishable, adding a quantitative analysis makes the distinction objective and measurable.
Like we did with the gasoline samples, we can overlay the chromatograms and do a quick visual comparison. These samples are much more distinct, as diesel sample 2 (red) is heavier. However, if we wanted to put these visual differences in more objective numeric terms, we can analyze these like we would a diesel sample by quantifying the saturate, mono/di/tri/tetra aromatic, and PAH content. Again, looking at the data, we’re easily able to distinguish these samples, and unlike a subjective chromatographic visual comparison, we can put hard numbers on these samples for distinction.
Analysis of Non-Motor Fuel Accelerants
Of course, arson can be committed with more than motor vehicle fuels from gas stations. There are plenty of other flammable liquids available, and it would make sense that they would have radically different compositions than fuels. Let’s take a look at a couple of examples – charcoal lighter fluid and paint thinner.

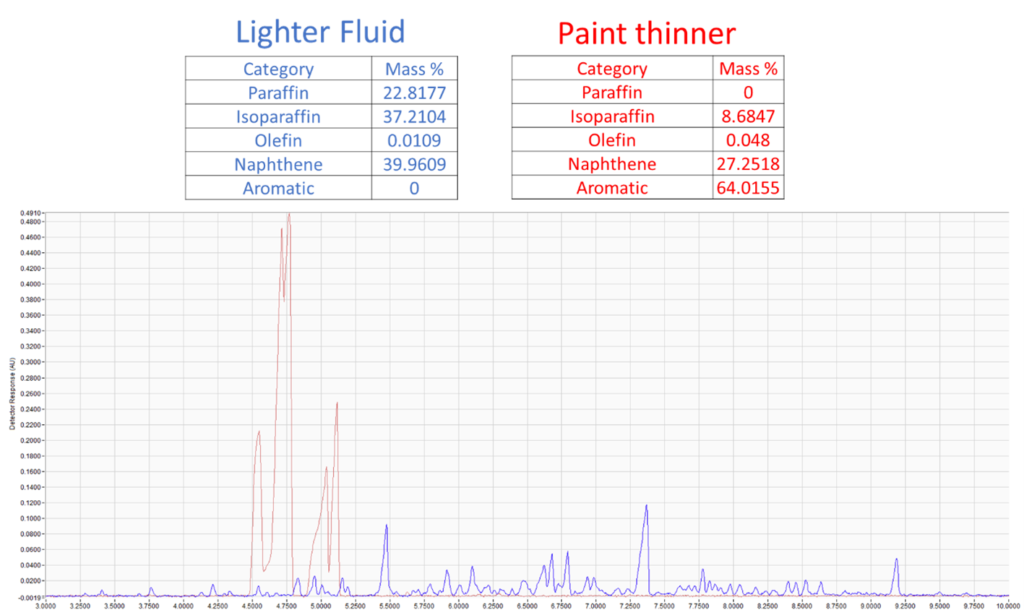
Figure 3. Comparison of PIONA content of lighter fluid and paint thinner extracted from simulated fire debris (top). Chromatographic overlay of the lighter fluid and paint thinner extract (bottom). The clear visual differences in sample composition are reflected in the PIONA analysis.
As you can see, these samples have very different compositions from one another and can be easily distinguished. Still, we’re able to apply a PIONA analysis to each of them and provide an objective, numeric distinction between the two. We can even take this a step further by spectrally identifying the peaks:

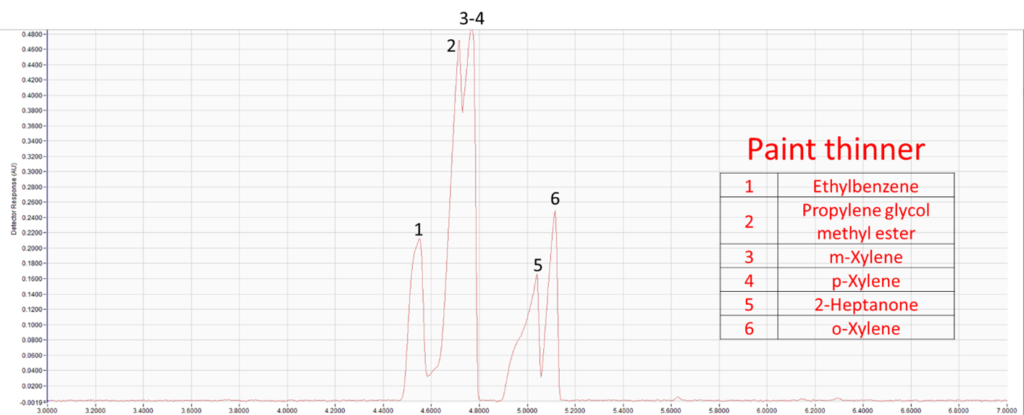
Figure 4. Identified components in the paint thinner extract. Like with GC-MS, GC-VUV can be used to identify individual compounds in suspected accelerants or ignitable liquids.
Although GC-MS is the most common method of analysis for fire debris samples, GC-VUV can provide crucial details that may fill in the gaps left by GC-MS. Vacuum ultraviolet spectroscopy’s strengths lie in its ability to spectrally deconvolve coeluting compounds and classify those compounds by their spectral shapes. Additionally, because of GC-VUV’s roots in the petroleum industry, hydrocarbon-based samples like fuels can be easily characterized using the same analytical power used in methods like ASTM D8071-19 and ASTM 8267-19. Spectral identification of individual compounds using GC-VUV adds even more details for investigators to use. Supplementing an arson investigation with the utility of GC-VUV may prove to be the perfect tool to aid arson investigations.
Have a question about fire debris analysis with VUV? Drop us a line below.










When I run this analysis, I use headspace to
avoid solvent interferences.
Is there any reason you used methylene chloride ?
Hi Raul, thanks for reading through the post! I agree, headspace is a great option for fire debris analysis. I chose to do a solvent extraction following ASTM E1412 because it would yield a liquid sample which I could analyze on a type-1 nonpolar column. We do have a headspace unit in our lab, and I think it would be a great idea to try the analysis again using headspace.
I used methylene chloride because it is a good, general-purpose nonpolar solvent, but other nonpolar solvents like CS2 would also work well.