Published Alex Hodgson on December 5, 2018
Part 1: Taking the Jet Fuel Industry Off Autopilot
I hope by now we’ve shown GC-VUV’s remarkable ability to analyze gasoline-range fuels by PIONA class using ASTM D8071. The natural progression is to the middle distillates, with the next heaviest cut being jet fuel (C8-C18 for Jet A versus C4-C12 for gasoline). Even this slight increase in carbon number increases the complexity of the sample multiplicatively and impacts our ability to confidently class-type all analytes, which means dividing them into saturates, olefins, aromatics and naphthalenes. In Part 1 of Flying at the Speed of (Ultraviolet) Light, we’ll discuss the current methods for analyzing jet fuel, and why it’s high time for a change.
Naphthalenes, which contribute heavily to soot formation during combustion, are monitored in jet fuel using ultraviolet spectrophotometry (ASTM D1840), which has been around since the early 1960s. Fuel samples are diluted and their total absorbance at 285 nm is measured. However, this method cannot give any qualitative information on the sample, and its low absolute absorbance can lead to a relatively large error range.
D1840 must use 285 nm as its absorbance wavelength to eliminate any potential interference from mono-aromatics. However, looking at the VUV absorbance spectra of several naphthalenes, we can see the relative absorbance at the λmax (approximately 210-230 nm) is more than 10 times greater than at 285 nm (Figure 1)! Think about all the sensitivity that’s being lost measuring way out at 285 nm, not to mention the unique absorbance spectral fingerprints that would allow us to identify individual naphthalene species (Figure 2).
The remaining classes are measured using fluorescent indicator adsorption (FIA). FIA was developed in the 1940s and is still the primary test method for measuring saturates, olefins, and aromatics in jet fuel (ASTM D1319). Fuel samples are physically separated using silica gel fractionation, and the length of each cut, marked by fluorescent dyes, is measured with a ruler. However, the boundaries between the cuts are not always clean, and manual measuring adds another level of human error to the analysis.
Recently D1319 has come under serious scrutiny in what I’ve dubbed “Dye-gate 2018”. The most recent batch of dyes is not fluorescing properly in the aromatics region, which as you can imagine can make analyzing jet fuel rather difficult (aromatics composing 10-25% of the total volume). To top it off, the sole manufacturer of this dye no longer exists, and so far, the synthesis of the dye has not been successfully reproduced, which has the jet fuel industry scrambling to replace ASTM D1319.
We often live by the mantra “if it ain’t broke, don’t fix it”, but it’s 2018 (also…it’s broke). It’s about time for a more technologically savvy method. Can jet fuel be analyzed using GC-VUV? Well of course it can; otherwise this would be a very short series! In Part 2 we’ll discuss how we extended the VUV spectra library to cover the jet fuel range and in Part 3 we’ll look at some real jet fuel samples and see how GC-VUV compares quantitatively to legacy methods.
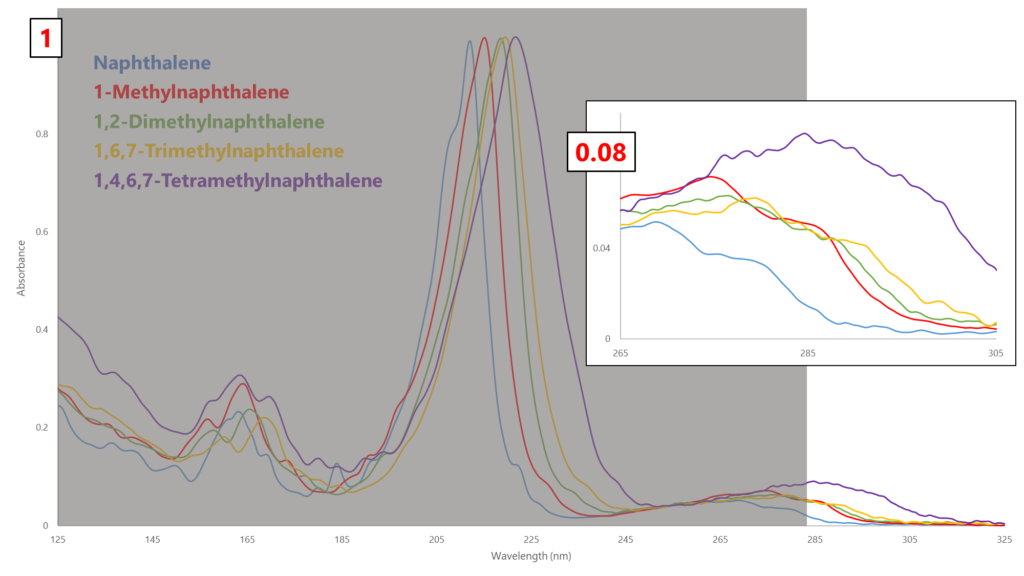
Figure 1. Look at how much absorbance is lost by measuring naphthalenes at 285 nm!
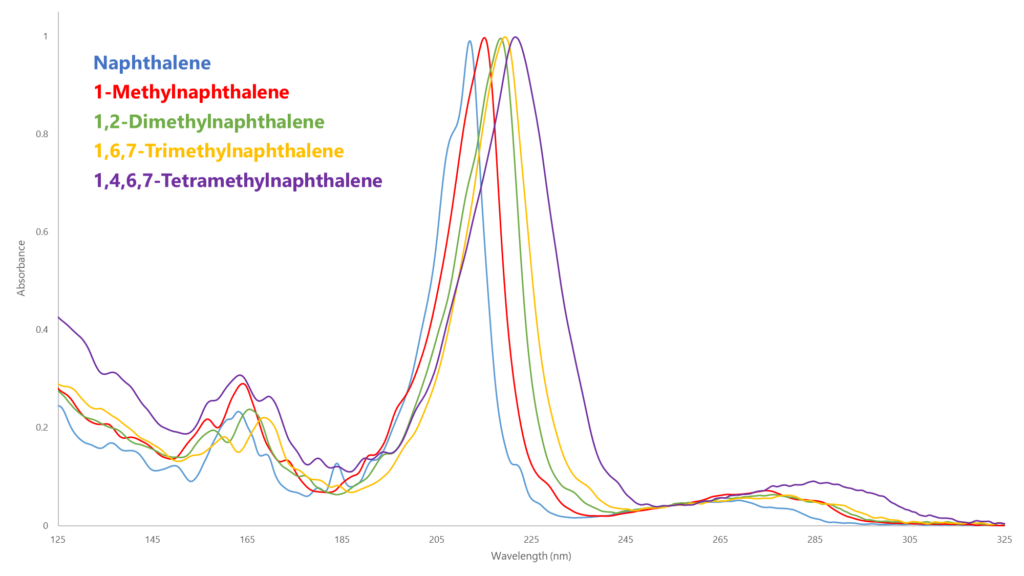
Figure 2. GC-VUV not only has much better absorbance around the λmax for each analyte (~ 210-230 nm), but the individual naphthalenes can be speciated during the normal quantitative analysis.








Leave a Reply