Published Ryan Schonert on November 13, 2019
3 important factors to consider before switching from helium in your laboratory
Every lab I’ve worked in has used helium as the carrier gas for their GCs. Most gas chromatographers will tell you that helium is the gold standard for gas chromatography, and with good reason – it’s safe, it delivers reasonable run times, and it’s inert. But let’s face it, at the end of the day, labs need to be profitable to survive, and helium shortages are hurting labs everywhere. Everybody needs helium, but there isn’t enough to go around; many labs have to deal with rising prices, and some are having a hard time getting any helium at all.
Thankfully, we already have some viable alternatives available: hydrogen and nitrogen. Both gases are relatively cheap to produce, and with a little work, we can achieve comparable peak resolution to helium. So why haven’t we switched already? Well, there are a few caveats we need to explore first.
RUN TIME
While each of these three gases – helium, nitrogen, and hydrogen – can achieve the same peak resolution, they achieve peak separation at different speeds. Take a look at this example in Figure 1, which shows a gasoline sample run using 3 different carrier gases.
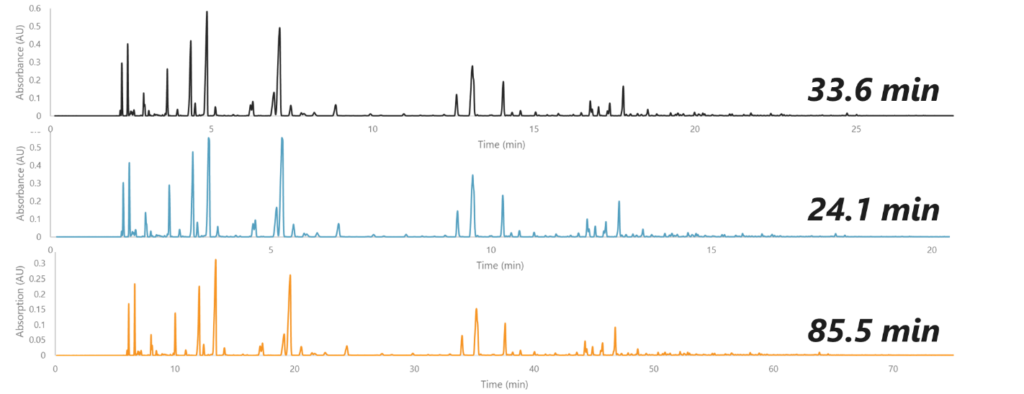
Figure 1. Gasoline sample run on helium (top), hydrogen (middle), and nitrogen (bottom). Using the different carrier gases, we can get the same chromatographic peak resolution, but we need different amounts of time to get this resolution.
The chromatography itself looks the same, but each gas took a different amount of time to achieve it. As we can see, hydrogen is the fastest, nitrogen is the slowest, and helium is in the middle. If we wanted to switch to nitrogen, we would need a lot more time for each run. Also, because our runs are longer, our peaks are broader and therefore shorter, causing a loss in sensitivity. We could forcibly speed up the run by altering the flow rate and oven conditions, which would shorten the run time and increase sensitivity, but that would cause us to lose resolution.
SAFETY
In terms of run time, hydrogen seems to be the clear winner. However, it has its own issues, the most pressing of which is safety. Unlike nitrogen or helium, hydrogen is flammable, which is especially concerning for our customers and partners in the fuels industry. Labs using hydrogen have to take special precautions to prevent explosions, especially when using cylinders of pressurized hydrogen.
DETECTOR COMPATABILITY
Not all detectors are able to use helium or nitrogen. For example, GC-MS works best with helium, but switching to hydrogen or nitrogen could cause problems. In the case of hydrogen, MS detectors (especially older ones) may have difficulty pumping down, which could adversely affect the quality of the mass spectra obtained. Nitrogen, on the other hand, has been shown to decrease sensitivity due to increased ion scattering and its ability to ionize more easily than helium.
So, what if you are running GC-VUV? Is there something we can do to mitigate any of these concerns that other detectors can’t? We wanted to find out, so here’s what we did:
First, we developed some methods to try out. We took three basic approaches:
- Pure translations – we took the current ASTM D8071 method conditions and ran them through a method translator to get conditions for nitrogen and hydrogen.
- Speed-optimized flow (SOF) conditions – we translated the method to nitrogen and hydrogen under SOF conditions, which give you a faster run time for a minimal loss in resolution. These SOF conditions significantly decreased the run times, especially for nitrogen.
- Forced ASTM D8071 run time – we used the method translator to give us nitrogen and hydrogen conditions with a forced runtime of 33.57 minutes
Second, we used these methods to analyze 6 samples under ASTM D8071 conditions. The relevant conditions altered by the method translations are listed in Figure 2.
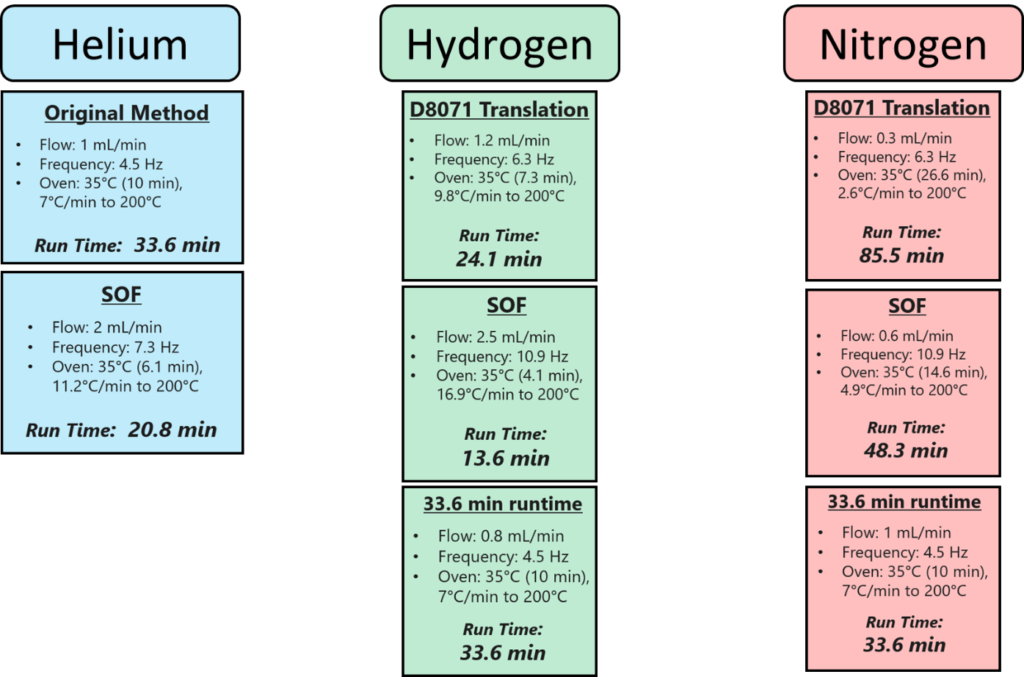
Figure 2. The relevant conditions changed for each method. The flow rate and oven programming were determined using a method translator. The acquisition frequency was determined by taking the number of scans in a traditional D8071 run and calculating a new frequency to give the same approximate number of scans.
The next two parts will focus on our results comparing helium vs. hydrogen and helium vs. nitrogen, as well as some advantages that GC-VUV has in this context. Stay tuned!








I work on VUV spectrocopy and trying to do GC-MS along with VUV on molecules.
A doubt regarding this comparison. Why not argon. Safe. Relatively cheap for 99.999 purity. Easy to procure and store. Inert like Helium (better than nitrogen which can react some of the molecules at high temperatures)). Run time will be high (compared to nitrogen). Why not varying sample delivery column diameter to length ratio to achieve lesser sample carrying gas times (Again per run any issue with amount of argon spent?cost factor?). Easy to pump (like nitrogen). No VUV lines in 125-225nm (like nitrogen)
Hi Dr. Rajasekhar,
As you point out, argon doesn’t have any absorption in the VUV region like nitrogen does, so the VGA detector could handle argon with few issues. We’ve even tested it as an alternative makeup gas for nitrogen in our lab’s VGA detectors. However, I believe it would be difficult to use argon as a GC carrier gas. The run times necessary to achieve good chromatographic resolution would be dramatically increased, enough so that argon is very rarely ever used as a carrier gas. VUV’s spectral deconvolution would allow you to sacrifice some resolution for a shorter run time, but speeding up the run too much could push the spectral deconvolution past its limits. Also, most GC EPCs aren’t set up to run argon, so even if argon was used, it would take additional work to tune the flow settings properly on the GC. I’m not aware of anyone using argon for GC-MS, although I’d suspect that would have additional issues.
Even though nitrogen does absorb in the VUV region, the absorption is small enough that it can still be used without trouble. At the start of a run, the VGA detector takes a reference scan which can be subtracted from the absorbance of sample compounds, so nitrogen in the flow cell is effectively ignored.