Published Jack Cochran on October 30, 2017
Alternative to Detailed Hydrocarbon Analysis
ASTM D6730 is the Standard Method for Determination of Individual Components in Spark Ignition Engine Fuels by 100-Metre Capillary (with Precolumn) High-Resolution Gas Chromatography. It’s affectionately known as Detailed Hydrocarbon Analysis (DHA) and is used for refinery quality control and product specification for gasoline fuels and blending stocks. The separation of gasoline compounds is highly efficient on the 100m x 0.25mm x 0.50µm dimethylpolysiloxane column, but a “tuning” 5% diphenyl-type precolumn is required for separation selectivity for certain aromatics and oxygenates, and the run time is 174 min (!).
In addition to determination of individual compounds, DHA is often used for bulk hydrocarbon group-type composition for gasoline samples, otherwise known as PONA or PIONA (Paraffins, Isoparaffins, Olefins, Naphthenes, Aromatics). I’ve recently been comparing DHA PIONA to PIONA results obtained with ASTM Method D8071, Standard Test Method for Determination of Hydrocarbon Group Types and Select Hydrocarbon and Oxygenate Compounds in Automotive Spark-Ignition Engine Fuel Using Gas Chromatography with Vacuum Ultraviolet Absorption Spectroscopy Detection (GC-VUV), for a wide variety of gasolines and gasoline-range samples. D8071 takes advantage of the powerful spectral deconvolution capability of VUV to produce a much faster run time of ~33.5 min on a 30m x 0.25mm x 0.25µm dimethylpolysiloxane GC column. What I’m finding, no surprise, is that ASTM D8071 with GC-VUV is very robust, and DHA with its hefty 3-hour run time and painful data review is not necessary for PIONA determinations when you have GC-VUV. Yay!
The D6730 and D8071 data comparison is for a future post. Like Austin, Texas today (October 28) where we had our first winter blast (the temperature dropped down to 37°F or 3°C!), I want to share some cool. While I was reviewing data from the early part of the DHA chromatogram for gasoline (Figure 1), I noticed the VUV absorbance spectra for certain trans- and cis- olefins are quite different (Figure 2), with the trans- isomers having distinctive sawtooth features in the 140-170 nm wavelength range.
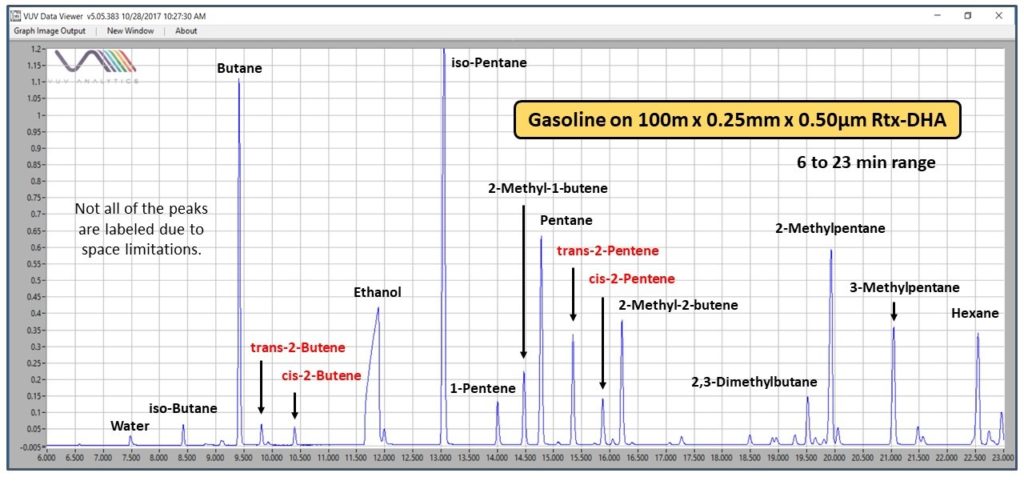
Figure 1. The first part of a chromatogram for E10 gasoline analyzed using ASTM D6730 GC conditions and a VUV spectrometer. The separation of the components is very efficient, including for the trans- and cis- olefin isomers highlighted in red.
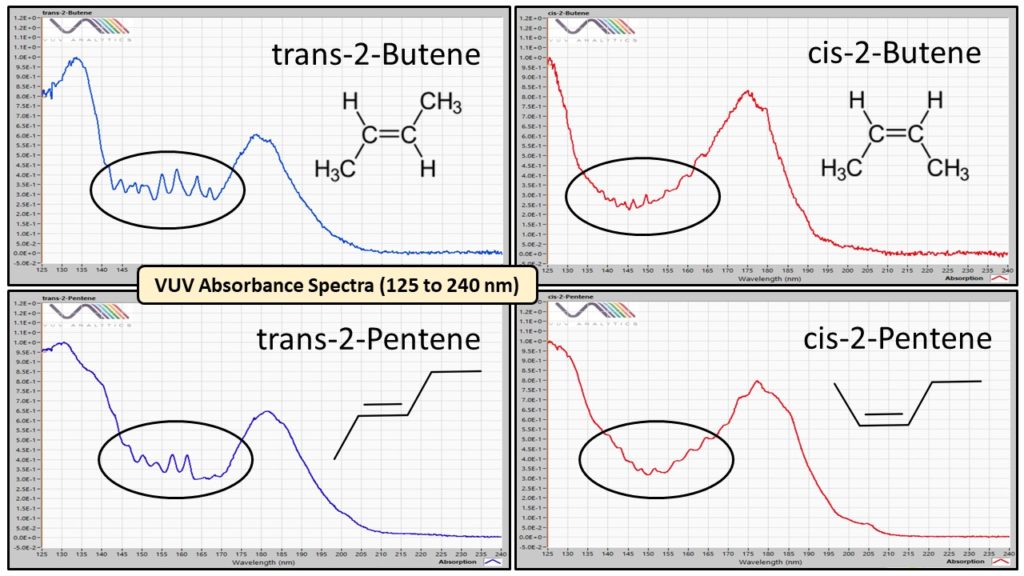
Figure 2. The vacuum ultraviolet (VUV) absorbance spectra for trans- and cis- configurations of 2-butene and 2-pentene are very different. The trans- isomers have interesting sawtooth features in the circled wavelength range. Spectral uniqueness allows deconvolution of components in case of their coelution.
These spectral differences for isomers are what gives VUV an advantage over mass spectrometry, where trans- and cis- conformations have identical electron ionization mass spectra (Figure 3). Distinctive absorbance spectra are what allow deconvolution for coeluting compounds, including isomers. While you can drive a truck through the separations of the trans- and cis- isomers in the Figure 1 DHA example, that isn’t always the case, especially when we use chromatographic compression to speed up analyses like we do for ASTM D8071.
I just can’t stop the wiggle in my spectrum…
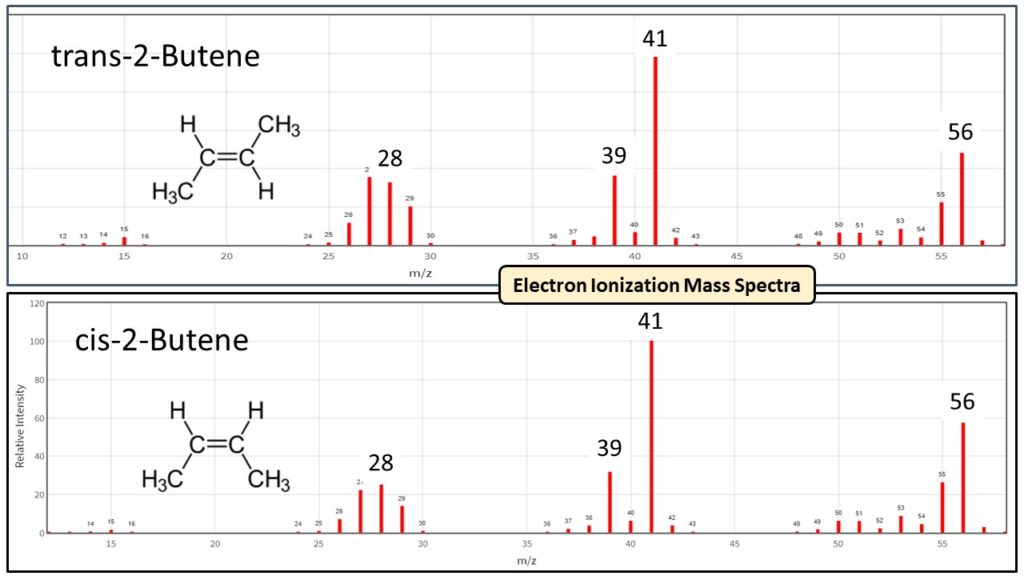
Figure 3. The electron ionization mass spectra for trans- and cis- 2-butene are essentially identical. GC separation is mandatory then when using mass spectrometry (MS) or flame ionization detector (FID) to determine trans- and cis- conformations of compounds.








Leave a Reply