Published Jack Cochran on July 3, 2018
Mixed Solvents Analysis Using GC-VUV
Solvent analysis using gas chromatography (GC) might seem simple, but when you have a non-specific flame ionization detector (FID) you might find yourself juggling column stationary phases to effect the obligatory separations. You could try mass spectrometry, but with small molecules like solvents, electron ionization spectra are indistinct and many of their m/z ions fall below the typical starting acquisition range. GC with vacuum ultraviolet (VUV) spectroscopy is an ideal alternate approach because VUV absorbance spectra are unique, especially for small molecules like solvents (Figure 1).
I did an experiment with 40 mixed solvents to demonstrate the advantages of GC-VUV, including: authoritative solvent identification, spectral deconvolution of coeluting solvents (including isomers), and automated data processing with accurate quantification. Figure 2 shows a chromatogram from GC-VUV analysis. Not all solvents are labeled, but I’ve marked some significant coelutions that were not resolved under any GC oven temperature programming conditions (2.5, 5, 10, and 20°C/min). Figure 3 zooms in on three of those coeluting solvent sets to demonstrate that quantification is not going to be possible, even with “creative” integration practices. Although a perpendicular drop at peak valley might allow an estimate for hexane’s concentration, the power of VUV spectral deconvolution will give accurate values, not only for hexane, but also for the other more significant coelutions.
Figures 4-8 show in stepwise fashion the power of using VUV Analyze and its push-button approach for automatically deconvolving coelutions and accurately quantifying the solvents of interest. As you page through those figures, notice how the colored vertical lines, which represent absorbance response, form the outline of Gaussian peaks for benzene, cyclohexane, tert-Amyl methyl ether, dioxane, trichloroethene, and isooctane. That’s an indication the deconvolution is working well. As you get to Figure 8 though, check out the yellow-lines peak shape for ethylene glycol. Ugly, right? Well, that’s what you get when you mix a very polar molecule (1,2-ethanediol; ethylene glycol) with a very non-polar GC stationary phase (Rtx-1; 100% dimethylpolysiloxane). The good news is that VUV Analyze tracks the ethylene glycol peak perfectly, even through its mid-point collision with cyclohexane.
The pictures are pretty, but the proof is in the numbers (Table 1). The coelutions of each group in the table shift slightly under different GC oven programming conditions, but VUV Analyze keeps up with the quantification task to produce good mass % values. For those forward-thinkers (and people who are impatient for good results), you can already see that one of the benefits of this approach is compressing the chromatography to get better sample throughput. More on that next time… For now, let’s get small in a big way with GC-VUV!
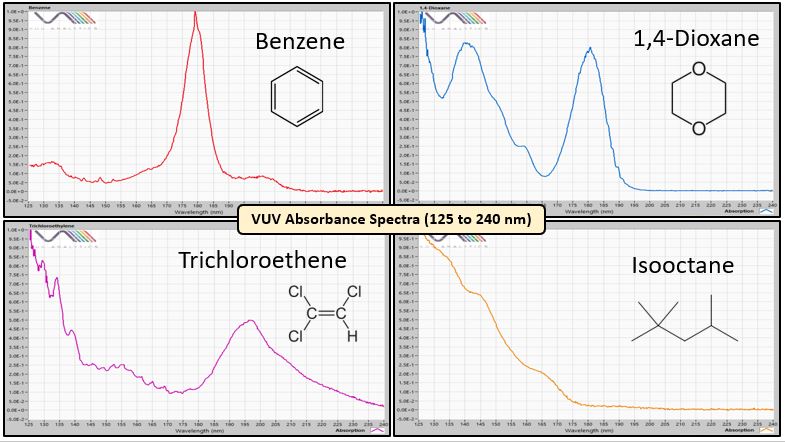
Figure 1. VUV absorbance spectra are unique, especially for small molecules like solvents, which leads to their easy identification and quantification.
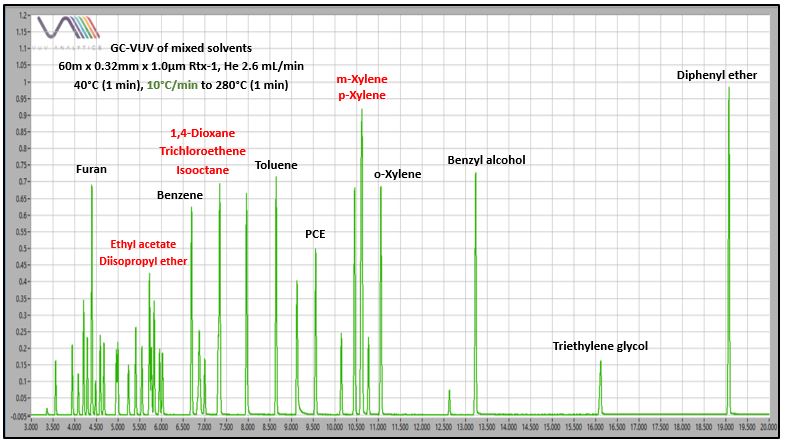
Figure 2. GC-VUV chromatogram of a complex solvents mixture. There are numerous coelutions that occur under all attempted GC oven programming conditions.
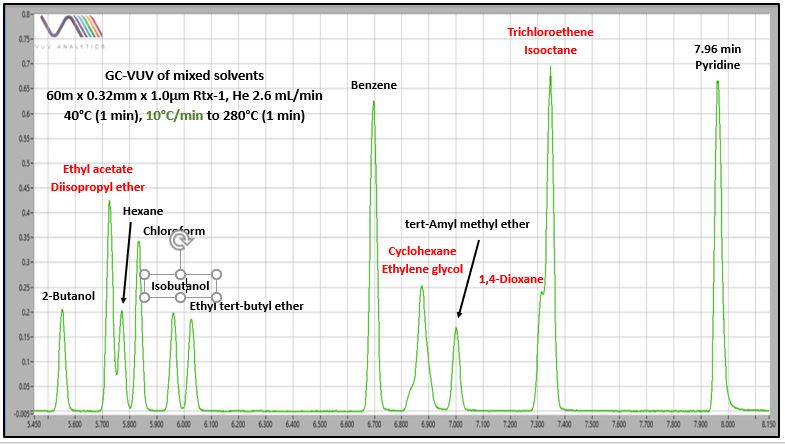
Figure 3. Zoomed-in GC-VUV chromatogram of a complex solvents mixture. Peak integration tricks alone will not allow accurate quantification for the coeluting solvents in red text.
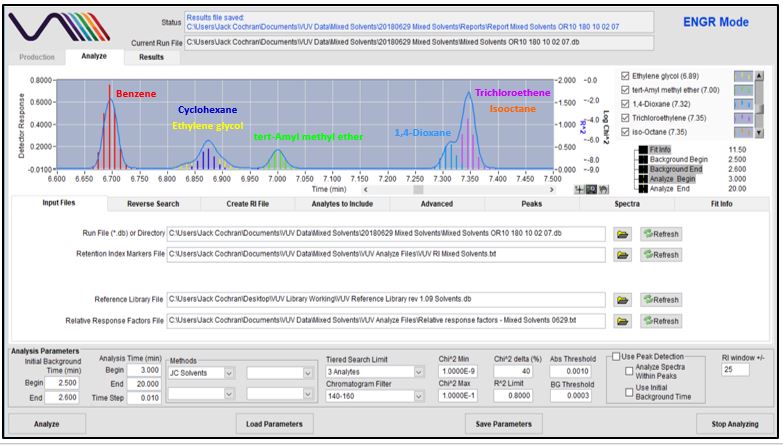
Figure 4
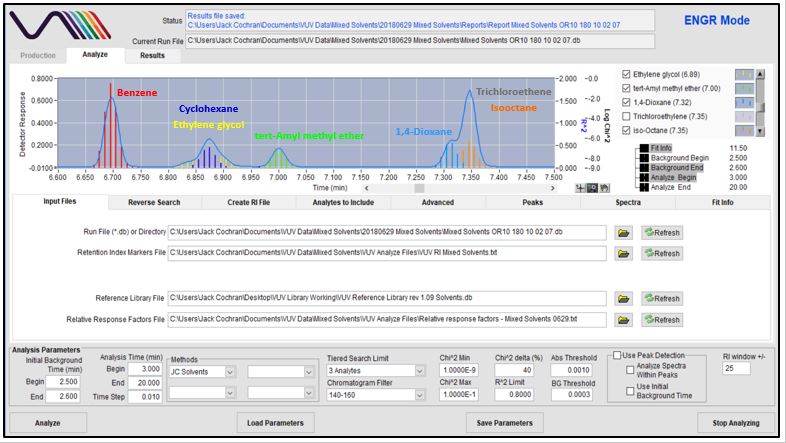
Figure 5
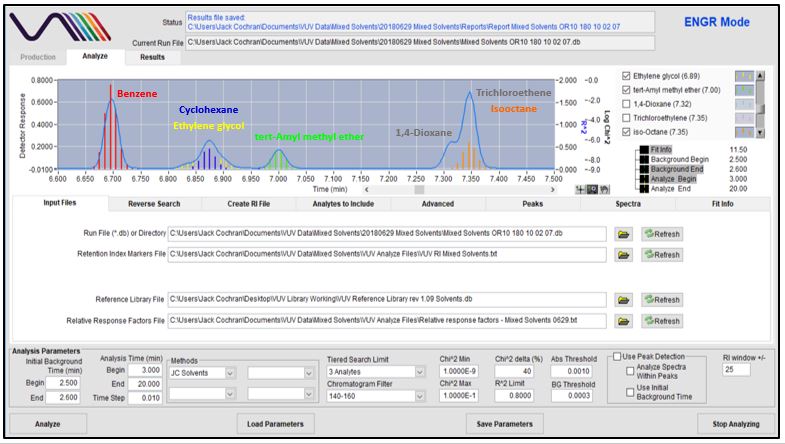
Figure 6
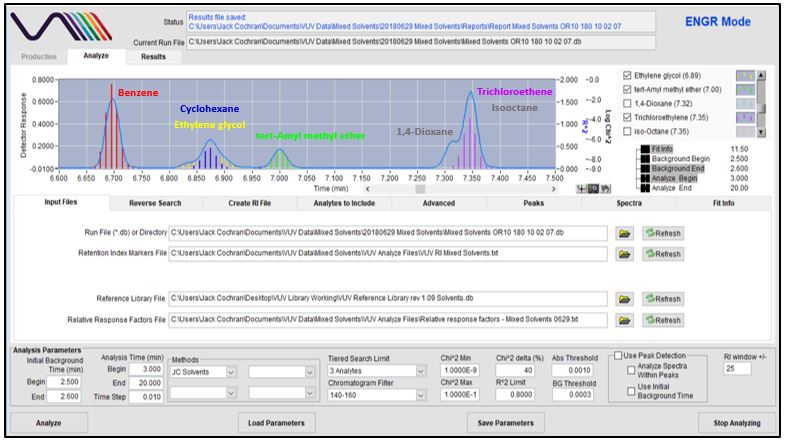
Figure 7
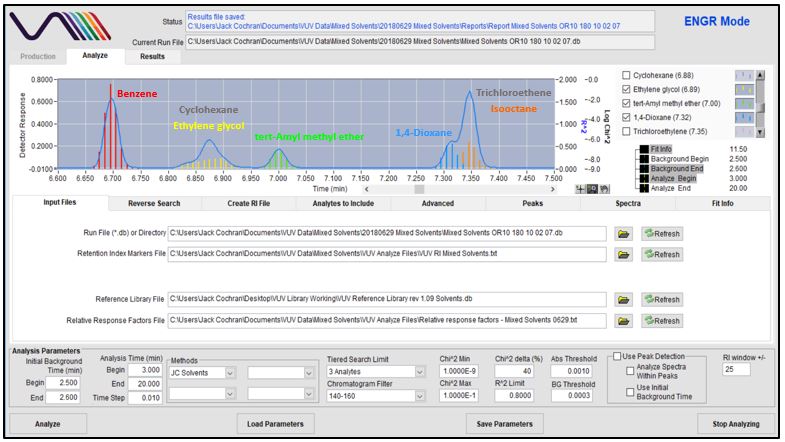
Figures 4-8. VUV Analyze offers a push-button approach to processing GC-VUV data. The series of figures shows how absorbance spectral deconvolution is used to assign unique responses to solvents for accurate quantification.
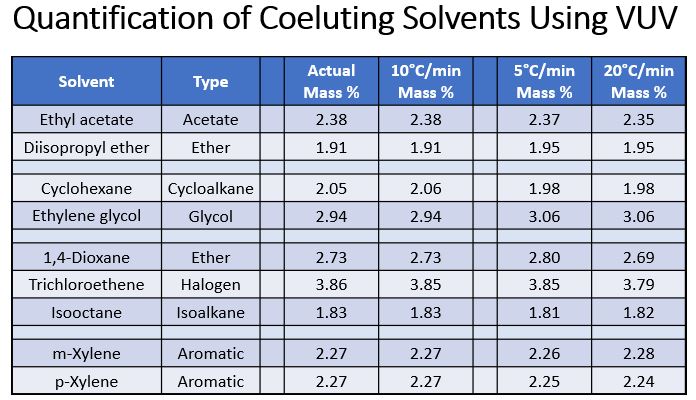
Table 1. Quantitative accuracy can still be achieved for coeluting solvents, even isomers, when using GC-VUV and VUV Analyze data processing.








Leave a Reply