Published Dan Wispinski on March 25, 2020
Carbureted engines seem a lifetime ago and today seem only one step ahead of Fred Flintstone’s foot-pedaled stone-wheeled sedan. Technological improvements by automakers have vastly improved the safety, efficiency and reliability of vehicles. Gasoline quality has also been vastly improved by sulfur, benzene, and vapor pressure limits. Fuel stability and cleanliness (through use of modern additives) are much better today. Olefins by way of their propensity to polymerize are still controlled by specification stability tests and are monitored by regulatory bodies. In the past, this polymerization often meant a messy and tedious job of cleaning gummy build up on fuel distribution components.
Olefin test methods used today are antiquated and more suited to the days of the carburetor. The ASTM D1319 FIA method is imprecise and the efficacy of the fluorescent dye at the core of the technique is in doubt. The ASTM D6550 method relies on instrumentation and consumables that are expensive to maintain. While both methods yield information on the total relative concentration of olefins neither provide insight into the molecular mass and type of olefins present – which is important. This may be why regulatory agencies are putting less emphasis on olefin monitoring; given relevance to modern gasoline.
The good news is that something better exists – some have even called it a revolutionary approach. I am of course referring to GC-VUV spectroscopy and ASTM D8071. This method not only replaces D1319 and D6550 for olefin analysis but also provides much more detail on a number of other important parameters..
A successful Interlaboratory Study (ILS) for D8071 determined precision for 13 gasoline parameters. The ILS sample set included ASTM gasoline proficiency test samples and SQS EPA Tier III samples with Accepted Reference Values (ARVs). Some of these samples were very useful to obtain ASTM D6708 statistical correlations to four EPA regulatory referee methods including the referee method for olefins. The successful ASTM D1319 correlation equation was:
Predicted Test Method D1319 = Bias-corrected Test Method D8071= 0.82373CD8071+0.5318
A comparison of the ILS olefin results for samples with ARV’s to ASTM D1319 is shown in Table 1.
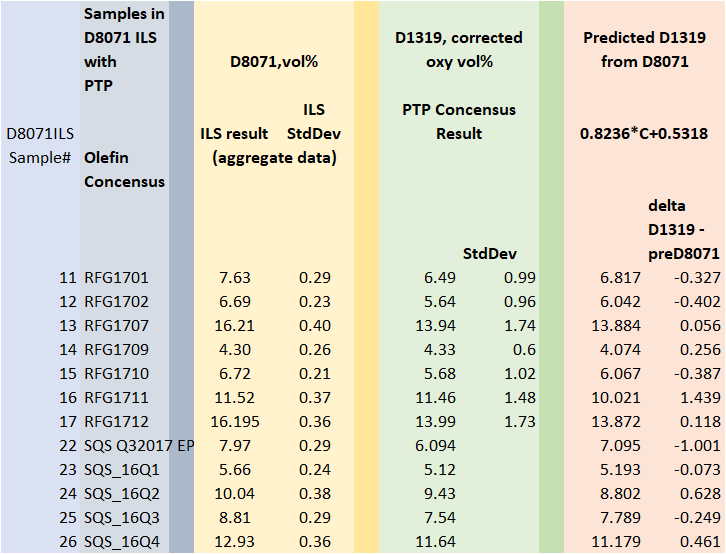
Table 1 – Comparison of Olefin results with ARVs to ASTM D1319.
The equation predicts the D1319 vol% olefins from D8071 olefin results in vol%. . It was developed in accordance with ASTM D6708 and conforms to EPA CFR 80.47 requirements. But what if the regulatory rules change? The EPA Streamlining (December 2019) exercise proposes D6550 in vol% for olefins. CARB regulations also cite D6550 with a conversion using sample density to vol%. CARB may change to olefin mass% and EPA may very well do the same. The ASTM D6550 method precision statement applies only to mass%.
In anticipation of a possible change to the regulatory olefin test method, a comparison of the D8071 ILS results for olefins (reported in mass%) to the results from D6550 was made. This comparison was done using samples that had D6550 consensus values. This approach, comparing PTP samples, has been used quite successfully to establish valid EPA correlation equations across many ASTM alternate methods.
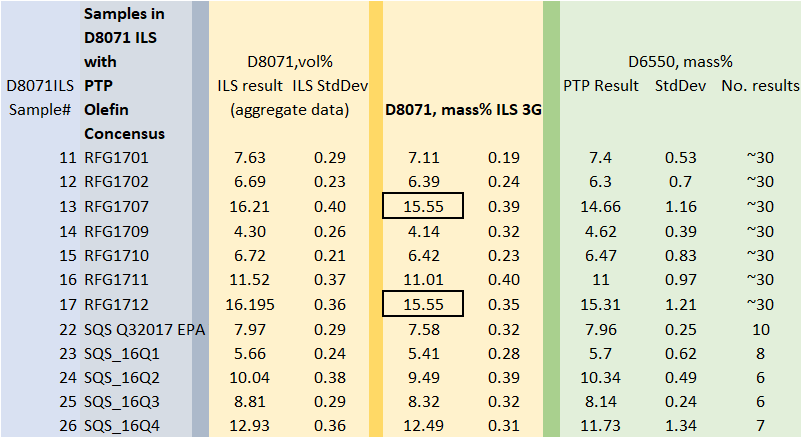
Table 2 – Comparison of Results across all samples.
Quality assurance protocols such as ISO 17025 and ASTM D6299 often use a double blind sample as a random performance check of measurement systems for audit purposes. Table 2 clearly shows the same consensus mass% values for samples RFG1707 and RFG1712 using ASTM D8071. A check on the results are shown in Table 3 below.
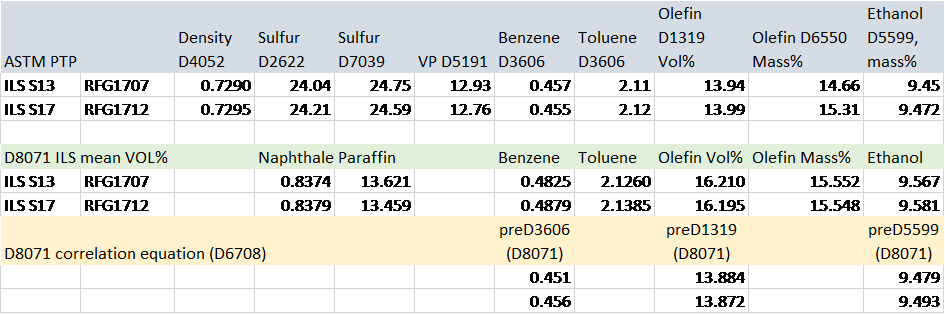
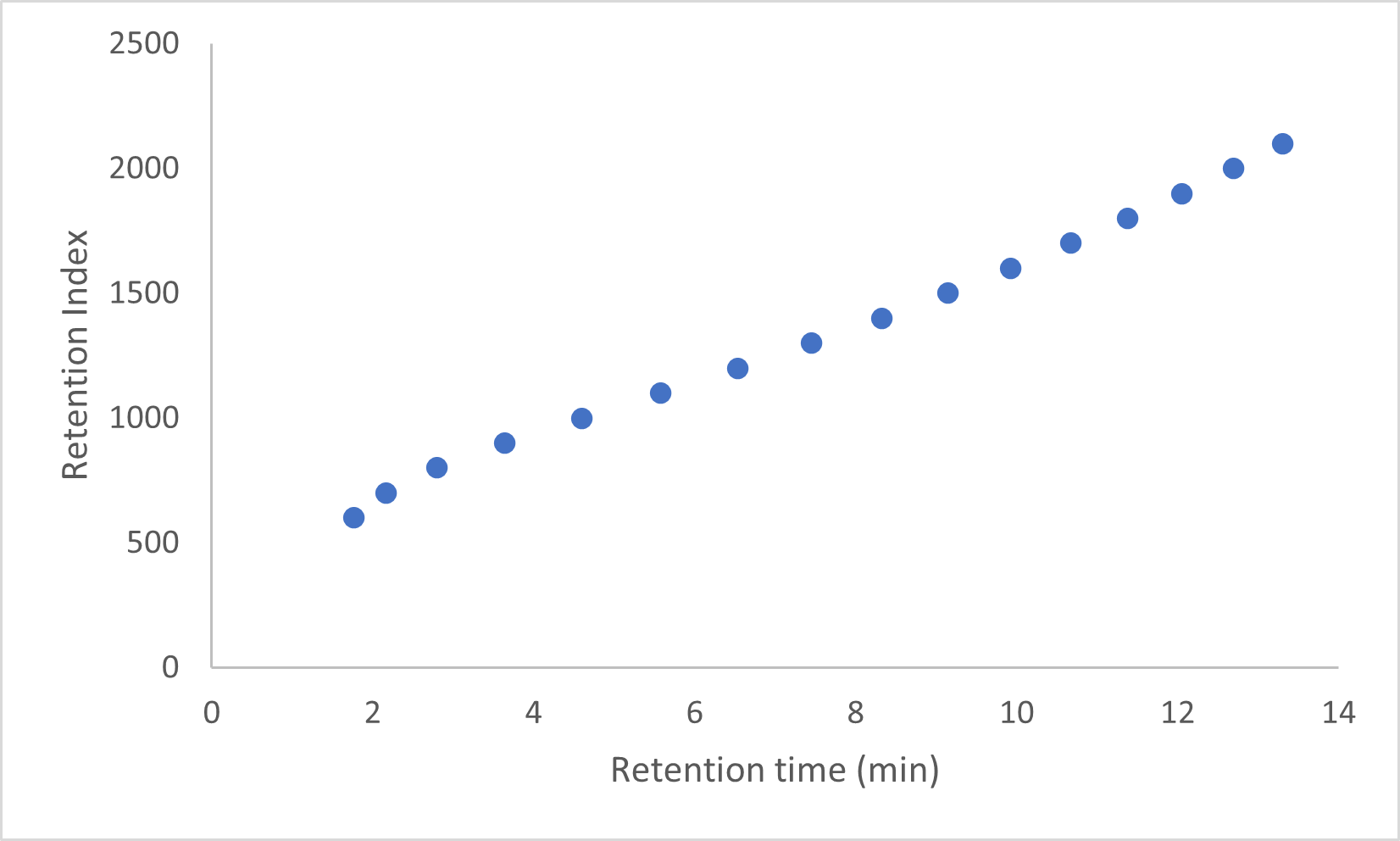
Table 3 – Comparison of Results for RFG1707 and RFG1712Two random chromatograms from the same participant of were chosen and overlaid. The chromatogram overlays below (Figures 1 – 3) in selected time regions (whole chromatogram, 1.7 to 7.5 min and 16.4 to 25 min) show identical responses.
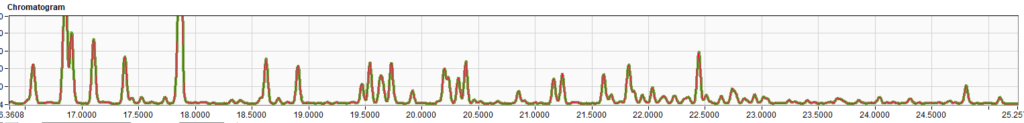
Figure 1 – Complete Chromatogram.
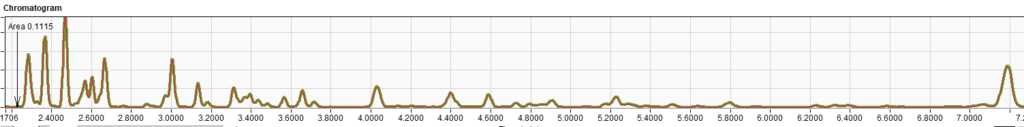
Figure 2 – Chromatogram selected time region 1.7 to 7.5 minutes.
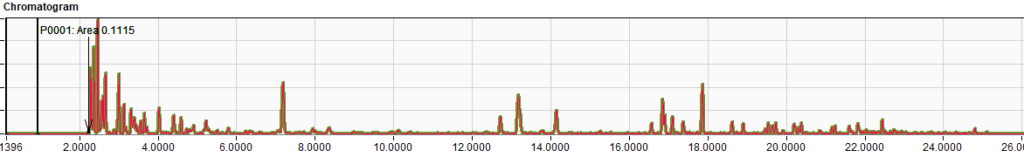
Figure 3 – Chromatogram selected time region 16.4 to 25 minutes.
ASTM D8071, as validated by the ILS, has shown that total olefins by the GC-VUV can be quantitated in mass and volume percent. A D6708 correlation equation from D8071 vol% to D1319 vol% olefins is published in D8071 and is compliant with EPA requirements. The D8071 olefin data shows that a correlation to ASTM D6550 is possible in either mass or vol%.
As stated earlier, the usefulness of total olefins may have limited value. Knowledge of the carbon number distribution and type of olefins is desirable and not available through other approaches. The power of GC-VUV enables the extraction of this detailed data from the absorbance versus time chromatogram. Figure 4 below , for one of the ILS RFG1707, samples shows time intervals (in green) for the deconvoluted olefin responses. This detailed olefin data can be used for correlation to bromine number and conjugated diene content. An appendix into ASTM D8071 is planned for these extensions. GC-VUV has left Fred Flintstone and ASTM D1319 in the dust – Yabba dabba doo!
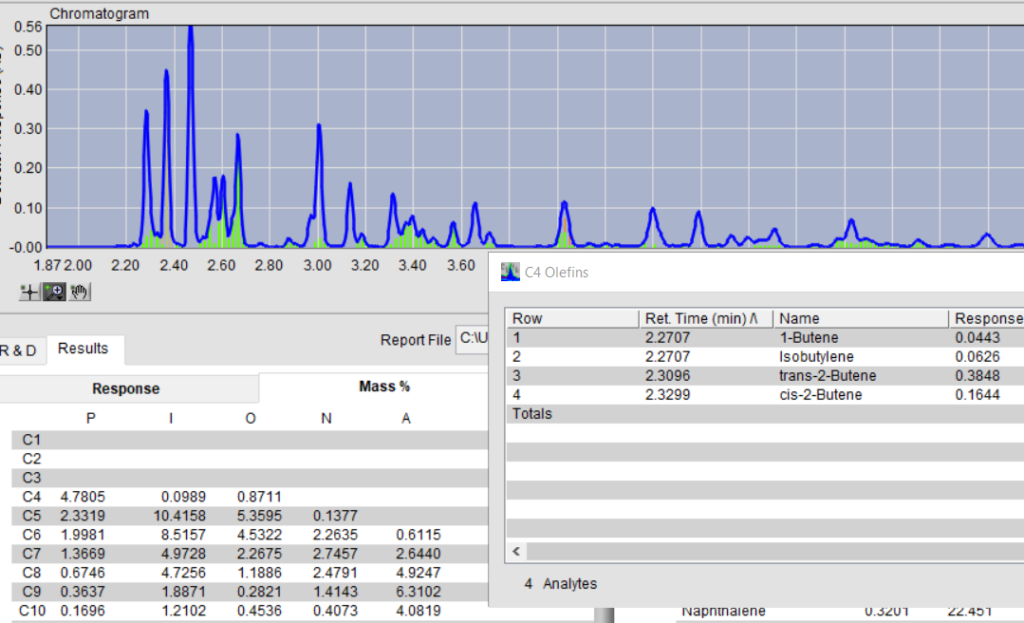
Figure 4 – VUV Analyze showing gasoline analysis with Olefin time slices.



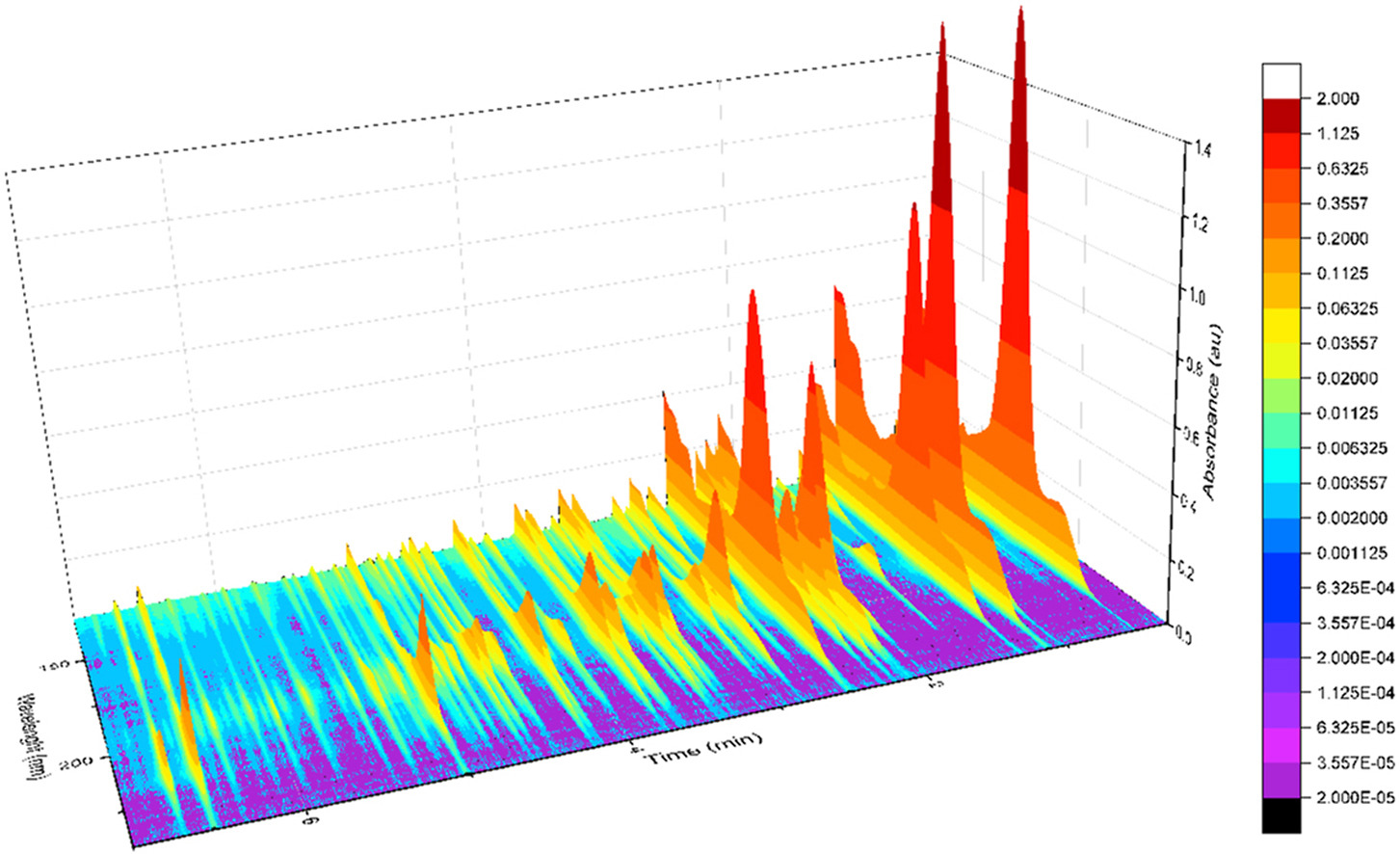





GC-VUV is an extremely tool to analyze complex HC samples.
However, GC is not the best technique to analyze any TOTAL. As we need to separate, identify ,calibrate, quantify and sum several (dozen) compounds, total deviation may be high.
To replace D1319, I think the best technique is HPLC. When I worked in INTEVEP(Venezuela) we developed an HPLC method, to analyze SOA(saturates, olefins, aromatics), with very good correlation against ASTM round robin samples.
Hi Raul,
Thank you for your comments, I have a few follow up questions:
Could you explain more about the technique that you developed? Are you referring to gasoline or are they confused with distillates? I am curious because I don’t know of HPLC methods that can separate olefins,
Would you like me to further explain about hydrocarbon classes that can be done by VUV because of their different spectral responses for hydrocarbon classes, D8071 focuses on hydrocarbon type with some speciation. Also, to my knowledge, I don’t think that HPLC can differentiate olefin type – conjugated vs. Non-conjugated, cyclic.