Published Alex Hodgson on May 3, 2017
Simplifying a Complex Sample Matrix….with Spectral Filters!
A complex sample matrix is the bane of analytical chemistry, and it’s why we go through such pains in sample preparation and GC method development to reduce the background interference and isolate our analytes of interest. It’s the proverbial needle in the haystack problem. And if you’re dealing with an unresolved complex matrix, or “hump-o-gram”? Forget it! Even if you can find your target in the mess, how do you integrate it? What baseline do you choose?
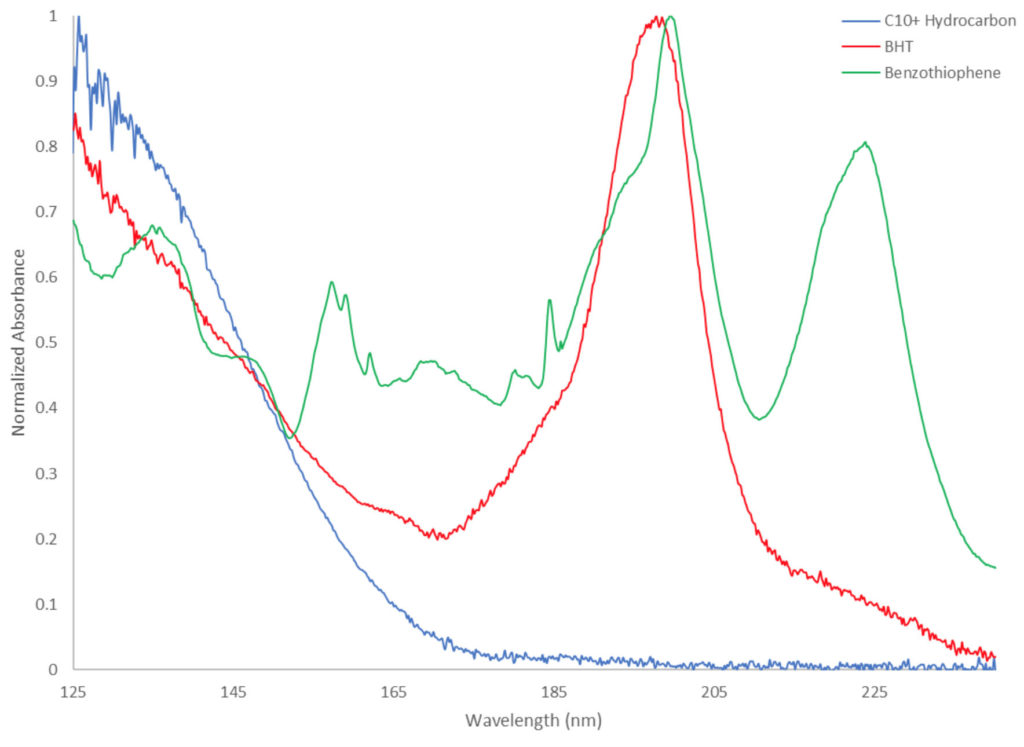
But what if, in looking for the needle, you had a shiny new pair of x-ray specs? Well if that “needle” is spectrally distinct, we can utilize spectral filtering to essentially eliminate our matrix background altogether, leaving our target isolated on a much less noisy background. What do I mean by “spectrally distinct”? Sure, each compound that absorbs in the VUV range produces a unique spectrum, but classes of compounds that share similar structural features will have similar spectral features. For instance, long (C10+) straight-chain hydrocarbons tend to have the highest response at 125-135 nm, with decreasing response to around 160-170 nm. Compounds with aromatic moieties show good response in the 170-240 nm range, such as 3,5-di-tert-butyl-4-hydroxytoluene (BHT) and benzothiophene (Figure 1).
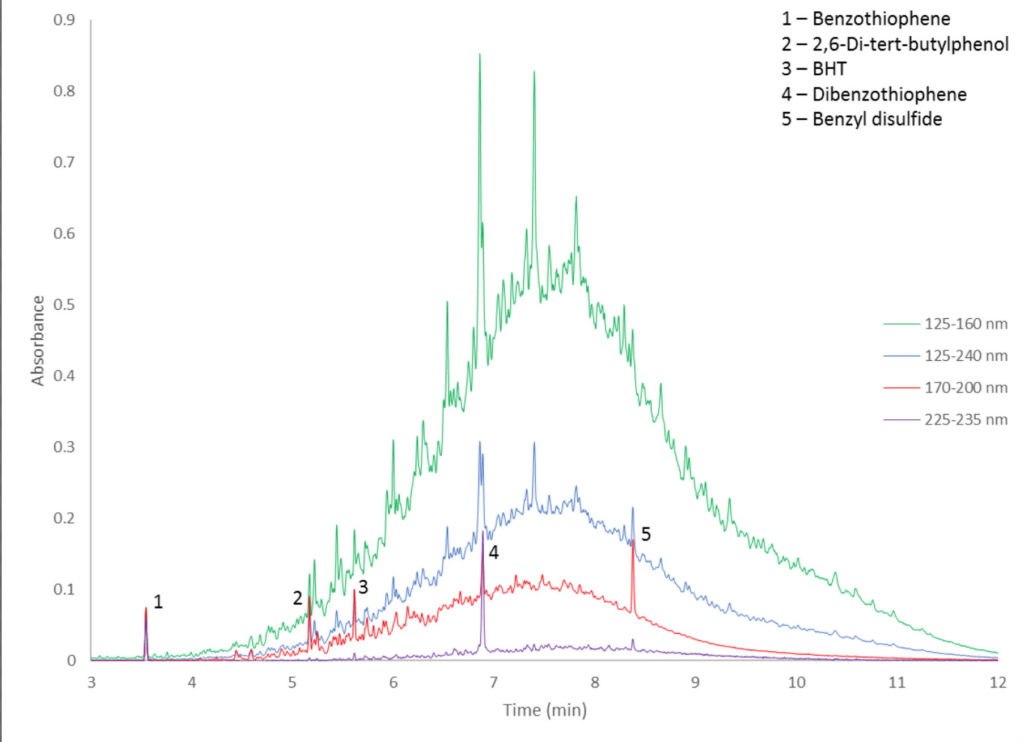
So why BHT and benzothiophene? These represent two major categories of compounds in transformer oils, the former being oxidation inhibitors and the latter being corrosive sulfur compounds. Figure 2 shows four spectral filters for the chromatogram of a 50 ng mixture of two oxidation inhibitors (BHT and 2,6-di-tert-butylphenol) and three corrosive sulfurs (benzothiophene, dibenzothiophene, and benzyl disulfide) in a 1:10 dilution of transformer oil. Just looking at the green and blue filters (125-160 nm and 125-240 nm, respectively), we would be hard pressed to reliably locate – much less quantitate – any of these analytes other than benzothiophene at 3.55 minutes. However, with a simple filter change (red filter – 170-200 nm, purple filter – 225-235 nm) the magnitude of the UCM is significantly reduced, leaving us with easily distinguished peaks. If we apply an even more focused filter, as with the 225-235 nm filter, the peak height of dibenzothiophene nearly triples!
While there’s no replacement for good chromatography, spectral filters are a handy tool when we’re trying to compress chromatography to shorten run times or, in the case of the hump-o-gram, when the matrix refuses to resolve at all. And because spectral filters are a data processing tool, we can create and add any number of spectral filters to the run file after the data is collected. This works much like an extracted-ion chromatogram in MS and potentially gives us an infinite amount of data from a single run!
Simple, right?







Leave a Reply