Published Jack Cochran on August 13, 2018
Styrene and Other Aromatic-Olefin Compounds
We take full advantage of VUV spectroscopy’s capability to classify small molecules based on their absorbance spectra in our GC-VUV approach for ASTM D8071, Standard Test Method for Determination of Hydrocarbon Group Types and Select Hydrocarbon and Oxygenate Compounds in Automotive Spark-Ignition Engine Fuel Using Gas Chromatography with Vacuum Ultraviolet Absorption Spectroscopy Detection (GC-VUV), which is PIONA for gasoline. Paraffins, isoparaffins, olefins, naphthenes, and aromatics have similar spectra within their respective classes, but what about combinations of those classes in one compound? Styrene and its companions as aromatic-olefins, molecules that are often present in pyrolysis gasoline, offer the perfect opportunity to answer this question.
Figures 1-5 show VUV absorbance spectra for styrene, 2-methylstyrene, 3-methylstyrene, 4-methylstyrene, and trans-beta-methylstyrene. It’s easy to see the class similarity for these compounds, and with a more careful review the reader can also determine the spectra are unique among each other. Unique spectra allow individual compound determination, if necessary, including the ability to deconvolve them should they coelute in the GC analysis, which does indeed occur for the 2-, 3-, and 4-methylstyrenes.
The aromatic-olefins are also easily differentiated from their aromatic-aliphatic analogs, as seen in Figures 6 and 7, especially where there is substantial absorbance in the 220-240 nm range for the styrene-type compounds. Class, style, pulchritude…whatever synonym you want to use, VUV spectra have it!
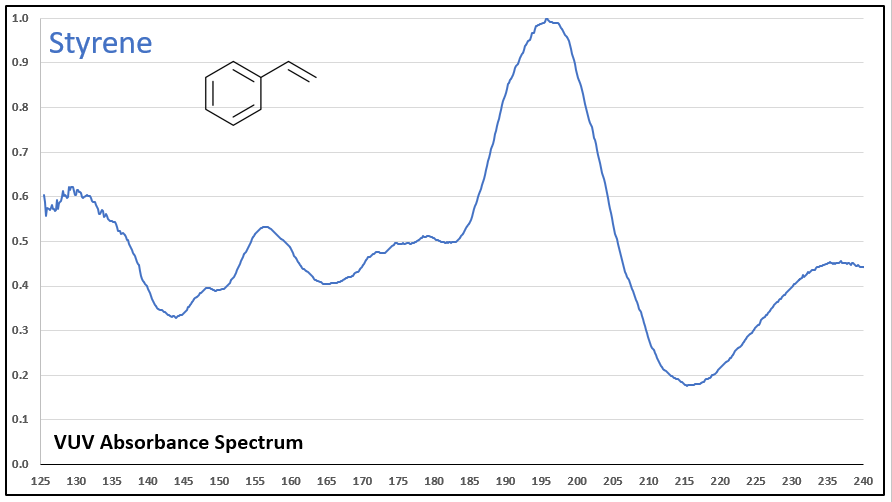
Figure 1.
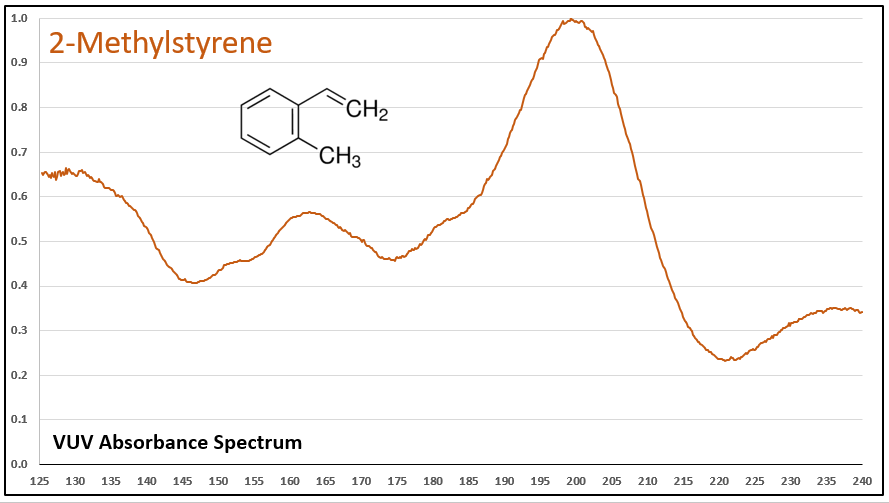
Figure 2.
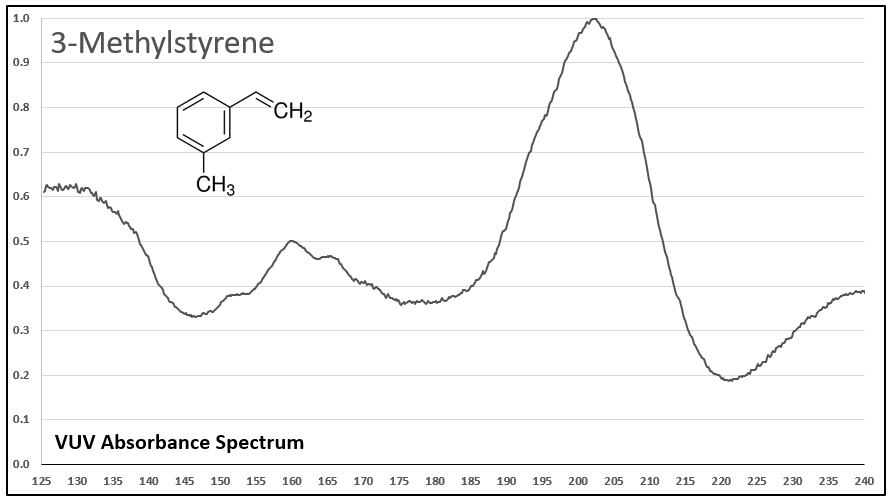
Figure 3.
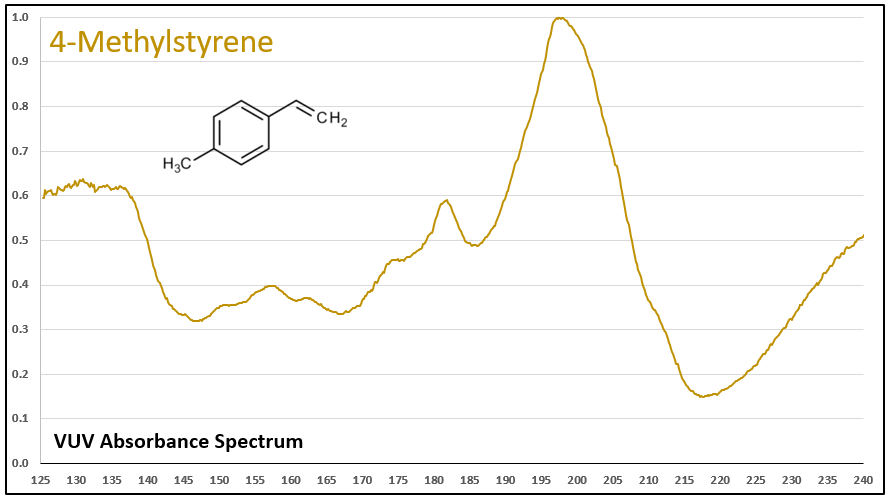
Figure 4.
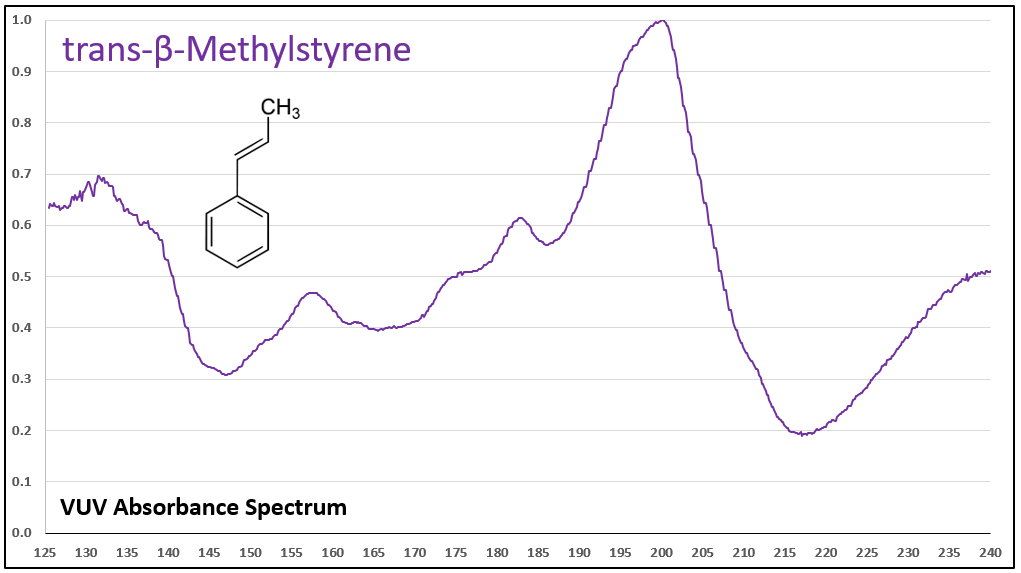
Figures 1-5. VUV absorbance spectra for styrene compounds, which have aromatic and olefinic character. They show unique VUV features in the 175-215 and 220-240 nm regions.
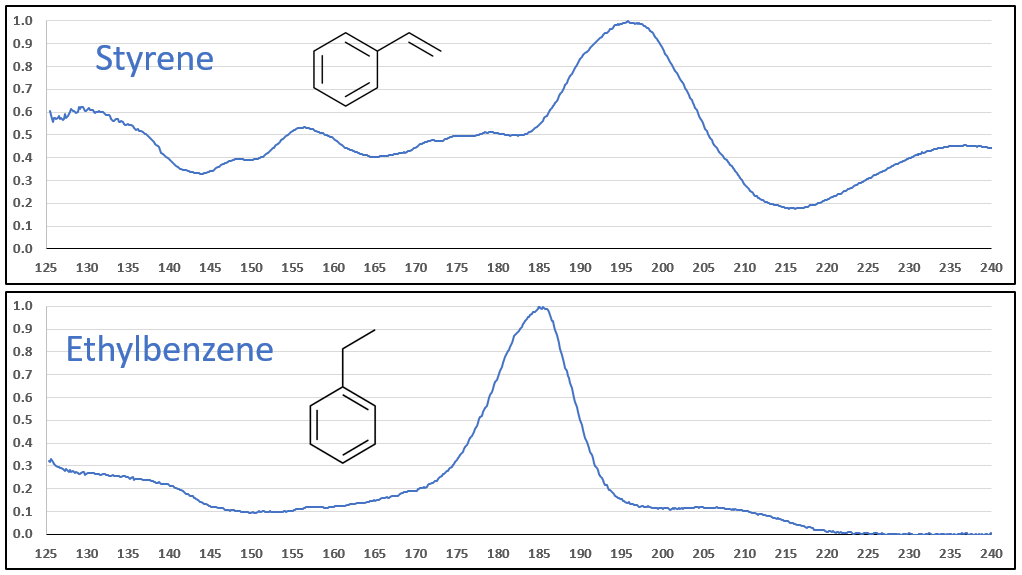
Figure 6.
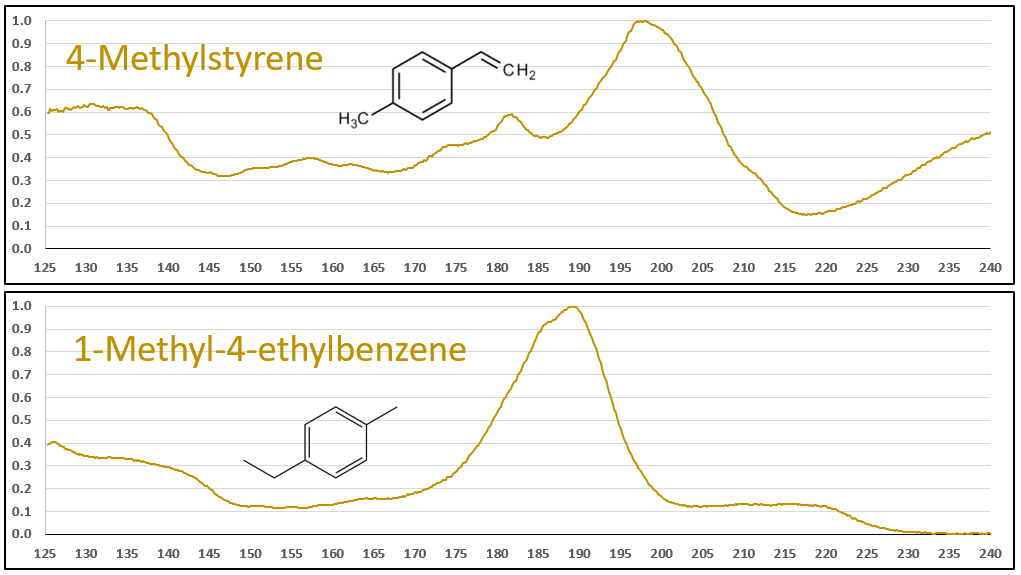
Figures 6 and 7. VUV absorbance spectra for styrene and 4-methyl styrene contrasted against their non-olefinic analogs, ethylbenzene and 1-methyl-4-ethylbenzene. The styrene spectra have their wavelength maxima shifted later (~185 > 195 nm), as well as showing substantial absorbance in the 220-240 nm range. These features allow interpretation (and classification) of styrene-type spectra in GC-VUV analyses of petrochemical samples.








Leave a Reply