Published Jack Cochran on March 27, 2017
Hopefully you read my last blog post on using vacuum ultraviolet (VUV) spectroscopy as a powerful detection means for gas chromatography (GC) of terpenes. If so, you may remember that where VUV shines (a little spectroscopy joke for you) is producing unique absorbance spectra for the various terpenes, even isomers. Unique spectra are what allow deconvolution of coeluting GC peaks, something I know a bit about from working with time-of-flight mass spectrometers. In mass spectrometry (MS), a (mostly) unique m/z ion for each coelutingcompound is what allows a mathematical routine to get going for deconvolution, followed by plotting apexing m/z ions for each coeluting peak to get its true spectrum. That logic has always been easy for me to wrap my mind around. It’s also simple to see how that same MS deconvolution approach fails for coeluting isomers since isomers have essentially identical mass spectra.
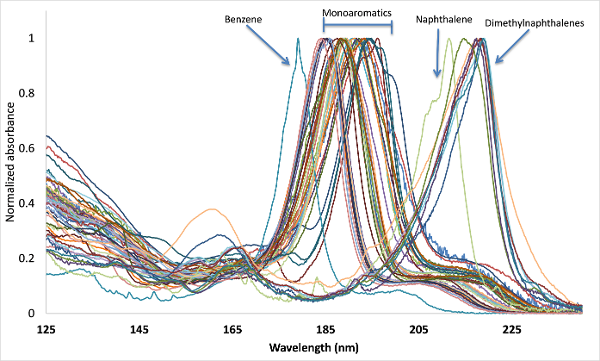
As a gas chromatographer using MS the coeluting-isomer problem didn’t bother me too much, as it allowed me to practice the art of separation. Inevitably though, critical compound separation leads to undesirable, longer GC run times, which brings us back to VUV and its uniqueness. Compression of the chromatography is achievable in GC-VUV for structurally similar compounds. What I wasn’t prepared for though when I joined VUV Analytics and starting reviewing absorbance spectra was how similar they could look to the untrained eye (Figure 1 – dimethylnaphthalenes). I remember starting a discussion on this topic with my colleagues Sean Jameson and Dale Harrison and they gave me polite smiles that seemed to say, “new guy”. I’ve since learned that every small “wiggle” in a spectrum not only signifies uniqueness, but is highly reproducible, which simplifies spectral deconvolution algorithms. In fact, for the dimethylnaphthalenes example, Kevin Schug saw it was possible to deconvolve isomers that had concentration differentials of 100. By the way, at least I recognized the advantage of similar absorbance spectra helping with qualitative identification of compounds and class filtration (the new guy wasn’t completely clueless!).
I think it’s important to finish by stating unequivocally that GC and MS and VUV are complementary techniques that can be used together to solve complex analytical chemistry problems. But I’m still quite proud to be associated with the only new one to come along in the last few decades!








Leave a Reply