Published Alex Hodgson on December 1, 2017
Terpenes in Essential Oils
Hey there. Rough day, huh? Why don’t you unwind a little bit? Whip up a glass of your favorite cocktail. Put on some smooth jazz. Draw a nice, hot bath. While you’re at it, why not add a few drops of an essential oil in there and really get that aromatherapy going. Which one did you go with? Tea tree oil? Excellent choice! There, that’s better. Now that you’re nice and relaxed, let’s look at some of the compounds that make that essential oil so delightfully fragrant in Part 3 of Better Living Through (Flavor) Chemistry.
Essential oils contain the extracted “essence” of a plant’s fragrance. They’re concentrated hydrophobic solutions of aroma compounds, typically on the order of milligrams – or even grams – per milliliter of oil. Most essential oils are naturally extracted from the plants themselves through processes like steam distillation, solvent extraction, and cold pressing.
So why should we care about analyzing these oils if they’re in such high concentrations? Clearly not to show off the sensitivity of the VGA-100. But instead what if we looked for something that wasn’t there? The compounds expected in these oils are well documented, so if we were to not find what we expect to find, it could call into question the authenticity of that essential oil.
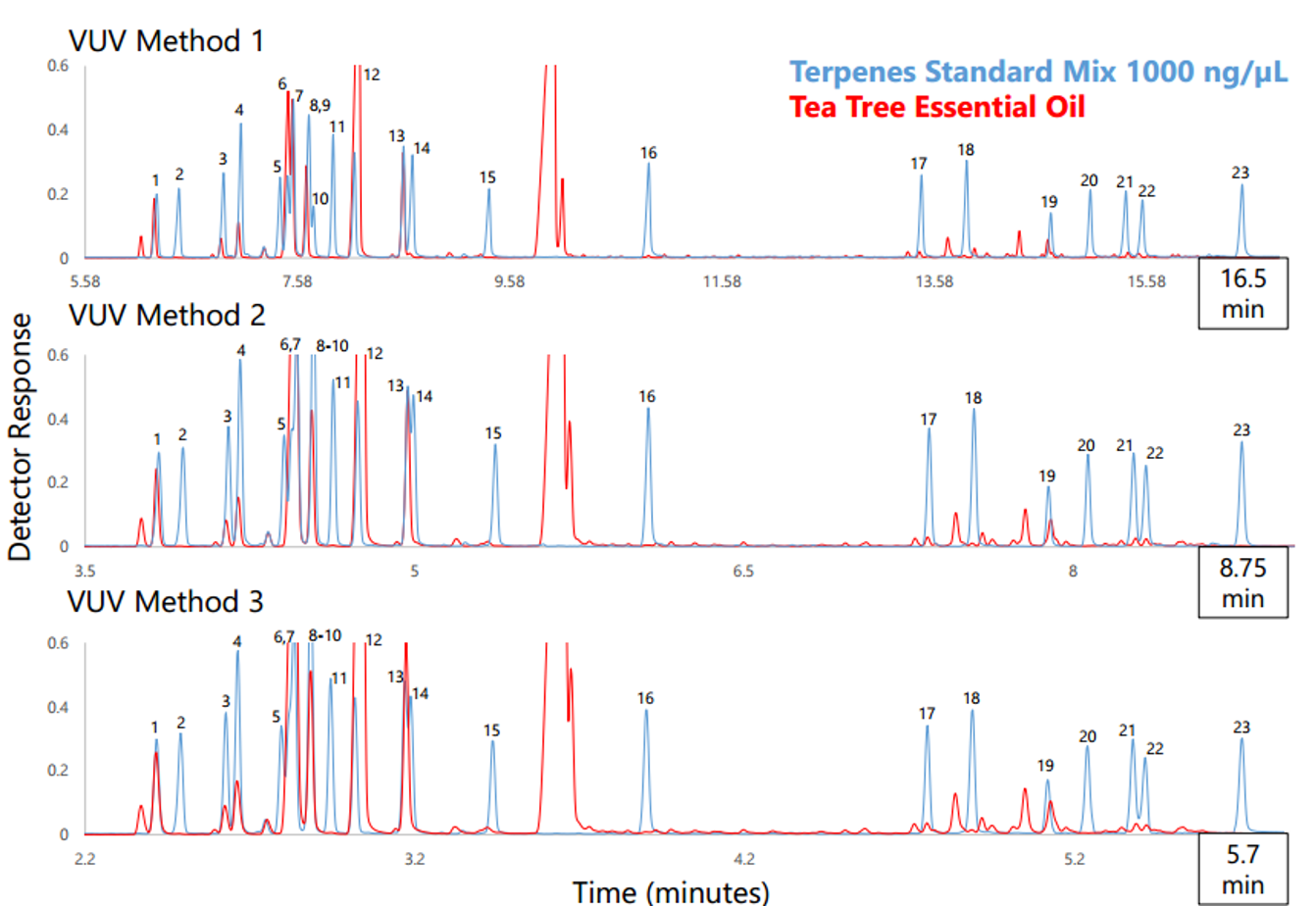
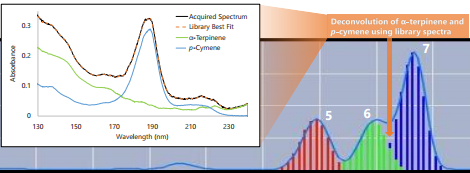
We used headspace-GC-VUV to analyze six different essential oils: eucalyptus, lavender, neroli, peppermint, sweet orange, and tea tree. Figure 1 shows the collected chromatograms for these six essential oils with the major peaks of interest labeled; the corresponding quantitative data are displayed in Figure 2 with the major peaks highlighted in green. And in case you missed how we can achieve such short run times and still accurately identify and quantitate, I cover the uniqueness of absorbance spectra and chromatographic compression in Part 1 and go through the spectral deconvolution process in Part 2.
In five of the six oils we see what we should expect. Eucalyptol in eucalyptus oil, cis- and trans-ocimene and linalool in lavender oil, menthol in peppermint oil, LOTS of limonene in the sweet orange oil, and α- and γ-terpinene in tea tree oil. However, the neroli oil seems to be missing some important terpenes. Where are cis- and trans-nerolidol? Note their absence both in the neroli oil chromatogram in Figure 1 and in the data table (highlighted red) in Figure 2. Both names, along with nerol, are derived from their original discovery in neroli oil, so you’d think these compounds would easily be found in any authentic neroli oil, right? Don’t let your nose fool you; all is not as it seems…or smells!
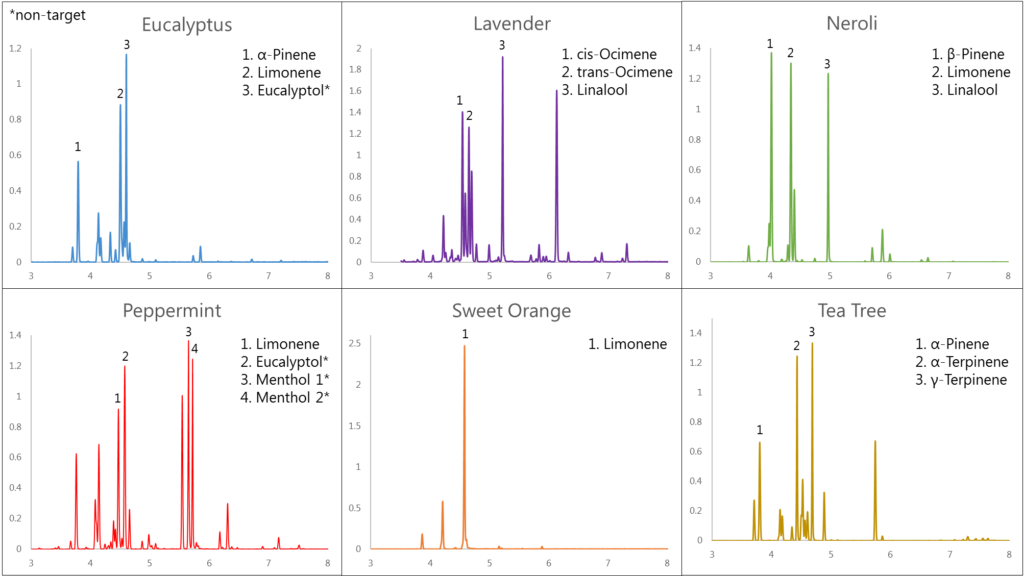
Figure 1. Essential oils can have one or several major terpenes that contribute to their characteristic odor profile. GC-VUV can easily identify these terpenes, even when they coelute with other terpenes, using their unique absorbance spectra.
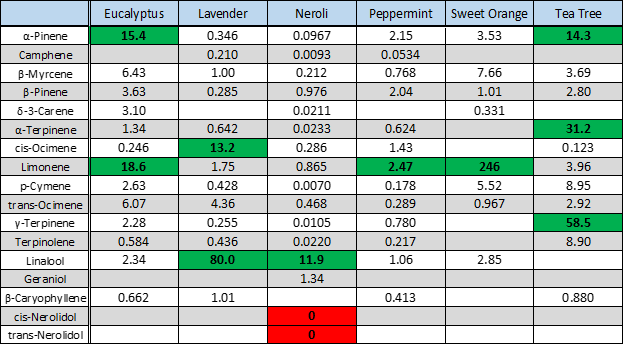
Figure 2. Quantitative analysis of essential oil terpenes. Key terpene concentrations of each essential oil (in mg/gram oil) are highlighted and bolded. Note the lack of cis- and trans-nerolidol in the neroli oil sample!
Next time we’ll shake up the way you think about your alcohol with a quality comparison of one of my favorite liquors: gin!
In case you missed it, check out part 1 and 2 of the blog series Better Living Through (Flavor) Chemistry:
Better Living Through (Flavor) Chemistry Part 1 – We Don’t Need No Separation, We Don’t Need No Flow Control
Better Living Through (Flavor) Chemistry Part 2 – Read Between (or Beneath) the Lines








Great post, I learn something totally new and informative on websites I stumble upon every day. Thanks for sharing.