Published Lindsey Shear-Laude, VUV Analytics on October 2, 2017
When analyzing pharmaceutical excipients and products for residual solvents using current USP static headspace-GC method conditions, a flame ionization detector (FID) is proposed and the corresponding GC analysis time is approximately 60 minutes. If a positive result for a residual solvent is detected it is necessary to run a confirmatory test using a different GC stationary phase (G16 type wax), which also gives a corresponding GC analysis time of approximately 60 minutes.
As an alternative to this methodology, VUV Analytics proposes the use of a VUV detector, which provides chemical information in the form of characteristic absorbance spectra for each analyte measured whereas an FID is non-specific. The VUV approach not only allows for high-confidence analyte identification without the need for time and resource-intensive confirmatory runs, but it also provides other key advantages, including faster GC run times (Figure 1) and the ability to deconvolute coeluting species (Figure 2).
The VUV detector can be operated using high GC carrier gas flow rates, which allows for significant chromatographic compression. For this static headspace application, we used a 30m x 0.25mm x 1.40µm Rxi-624Sil MS GC column with a constant He carrier gas flow of 4 mL/min. This combined with a 30oC/min GC oven temperature ramp allows for very fast GC, combined class 2 and class 3 residual solvent analysis. To maximize sensitivity at 4mL/min carrier gas flow, we operated at a split ratio of 2.5 while still maintaining 10 mL/min flow out the split vent, which is the Agilent recommended minimum.
The static headspace GC-VUV approach leads to combined solvent class analysis with a single GC run, good detectability and linearity for most Class 1, 2, and 3 solvents in pharmaceutical matrices at US-relevant concentration ranges, as well as fast analysis times to increase throughput.
See More in the Pharma Industry Page>
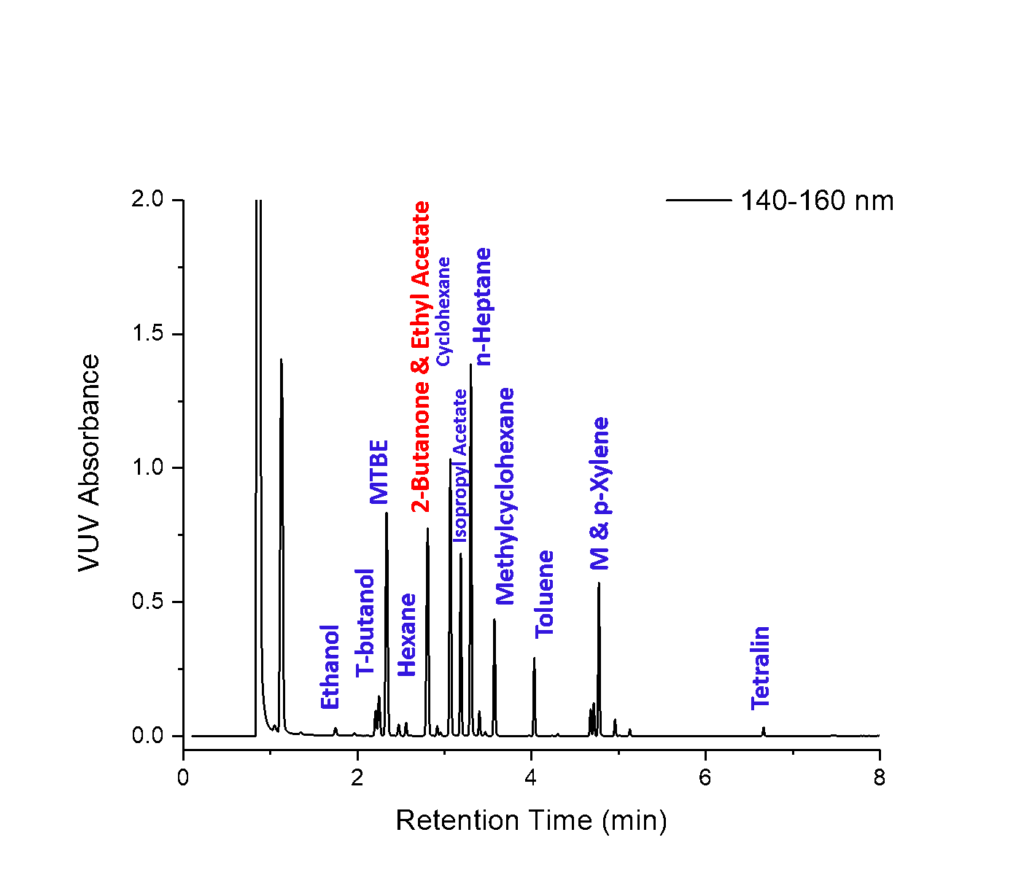
Figure 1. Static headspace GC-VUV of Class 2 and 3 residual solvents spiked in infant acetaminophen dissolved in water. The last compound, tetralin, elutes before 7.0 minutes. This ultra-fast GC-VUV analysis is made possible by using a He carrier gas flow rate of 4.0mL/min combined with a 30°C/min oven temperature ramp. The ability to deconvolve coeluting analytes is also possible using vacuum ultraviolet spectroscopy. A key coelution is highlighted in red. Overall, this methodology shows great promise for the analysis of residual solvents. Namely a single run which eliminates the need for dual column confirmation.
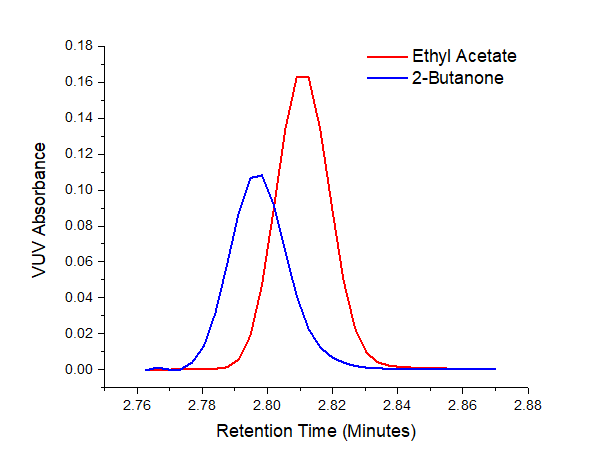
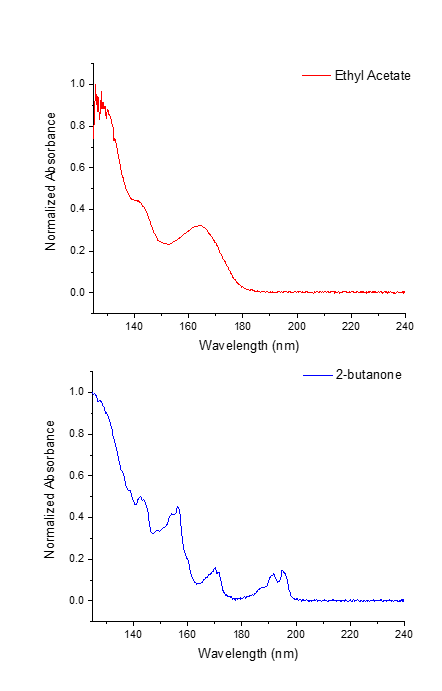
Figure 2. VUV spectral deconvolution of coeluting Class 3 solvents, 2-butanone and ethyl acetate, made possible by the uniqueness of their vacuum ultraviolet absorbance spectra.

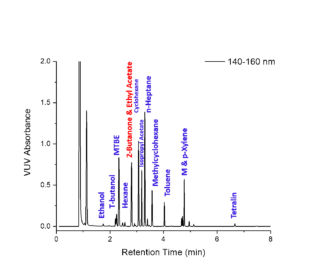





Leave a Reply