Published Jack Cochran on August 6, 2018
Using VUV Spectroscopy to Overcome GC Coelutions for Small Molecules
In my previous post I showed how small molecules in a homologous series have distinctive VUV absorbance spectra, using normal alkanes as the examples. The spectral differences in the 125-165 nm range for propane, butane, pentane, hexane, and heptane were obvious upon inspection. But I can show you some cases that are even more striking, like the spectra for primary alcohols (Figure 1).
A small organic molecule that contains an oxygen atom always seems to produce a feature-rich VUV absorbance spectrum. The methanol spectrum in Figure 2 clearly supports this observation. As alkyl tail length grows for an alcohol, the pointier features become suppressed (Figure 3) and eventually the alcohol spectra are quite similar in appearance (Figure 4). Gas chromatographers won’t care too much about that, given that these alcohols are easily separated from each other, but what is important is whether spectra have enough character to be deconvoluted from other coeluting compounds. That’s easy for one example that we sometimes see when running ASTM D8071 – methanol, butane, isobutane – because their spectra are so different from each other (Figures 5 and 6). GC-FID and GC-MS cannot tolerate these coelutions (and many others) because FID is non-specific, and for all practical purposes MS is too when dealing with such small molecules where overlap in m/z ions is very common. Get the right answer with GC-VUV!
Figure 1. Structures for a homologous series of primary alcohols from methanol to heptanol.
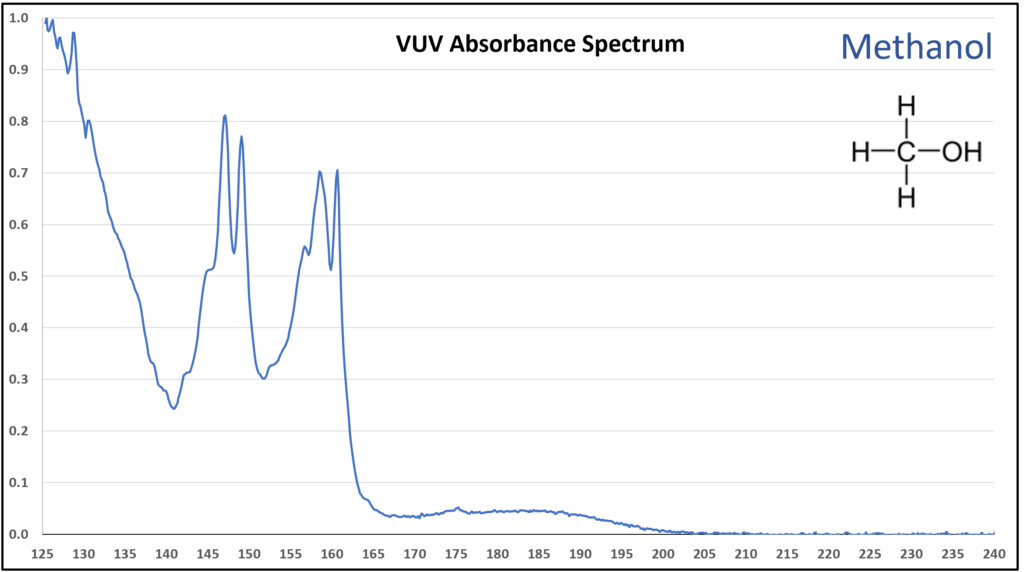
Figure 2. VUV absorbance spectrum (125-240 nm) for methanol. The spectrum is highly distinctive, which leads to easy identification and deconvolution from coeluting compounds.
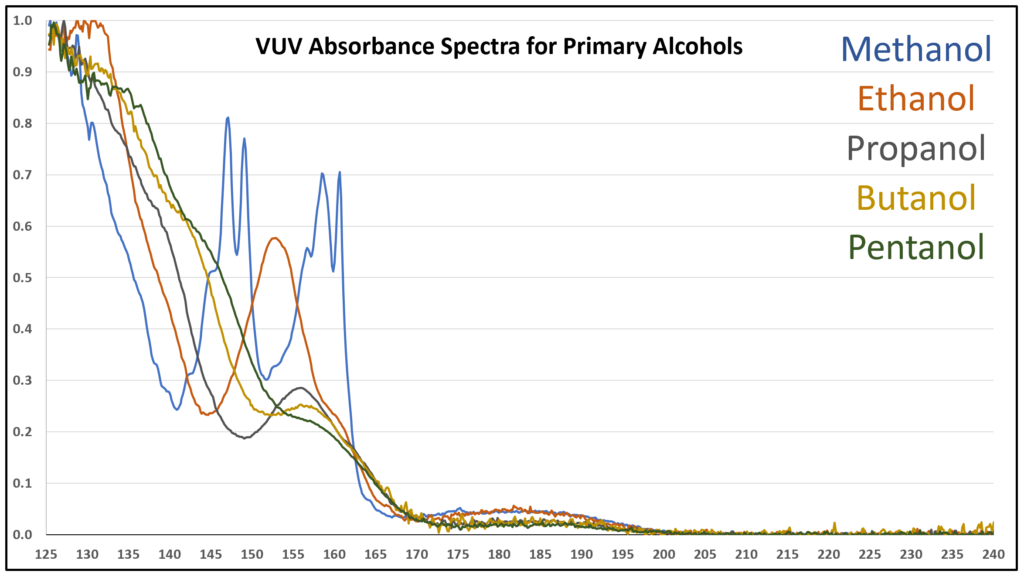
Figure 3. VUV absorbance spectrum (125-240 nm) for primary alcohols. As the alkyl tail grows longer, the spectra become less distinct, especially versus methanol.
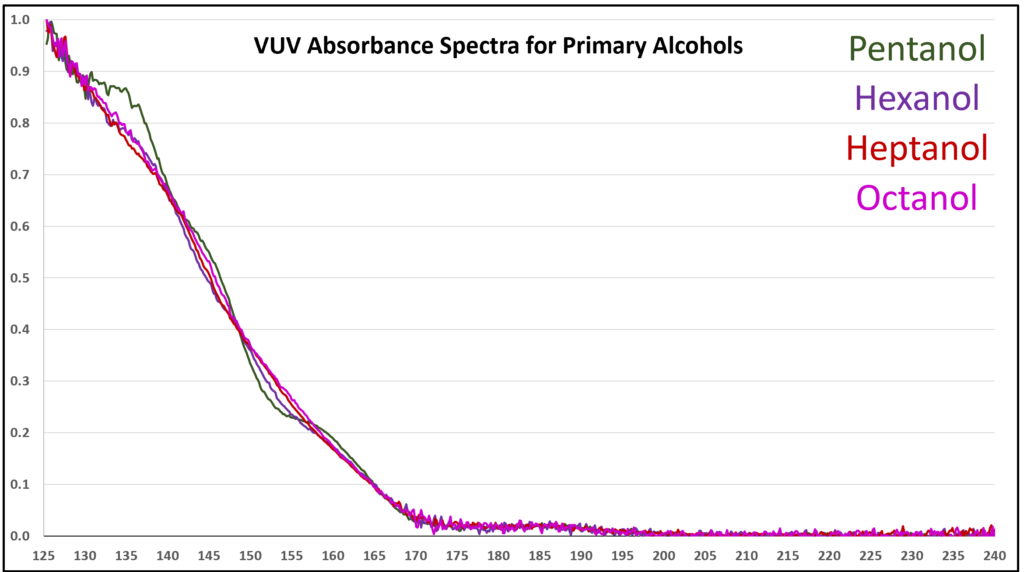
Figure 4. VUV absorbance spectra (125-240 nm) for higher primary alcohols. The spectra are becoming more similar, but these alcohols are easily separated by GC.
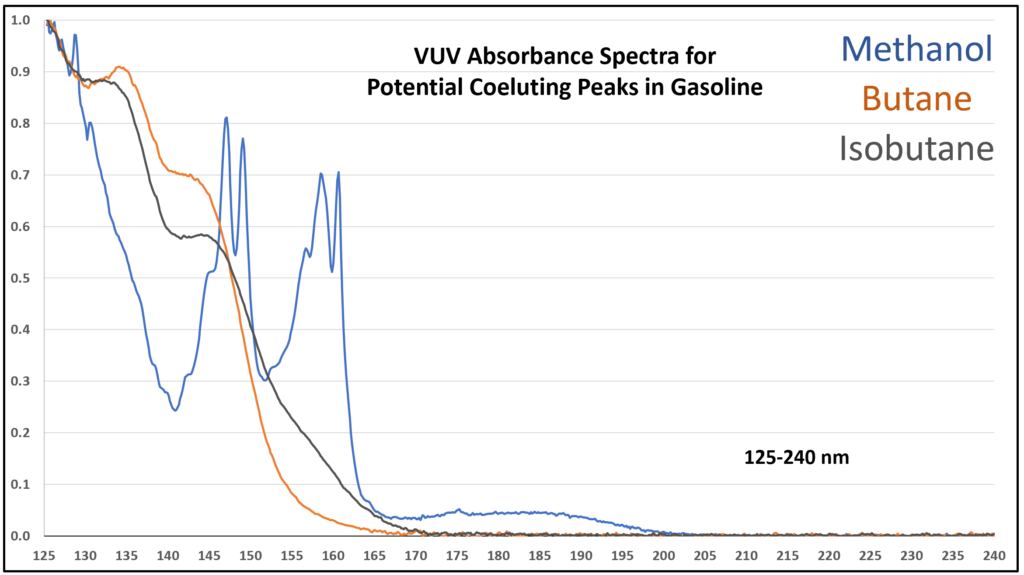
Figure 5. VUV absorbance spectra (125-240 nm) for compounds that can coelute in gasoline analysis using GC-VUV. Spectral uniqueness allows for easy deconvolution, which results in accurate identification and quantification.
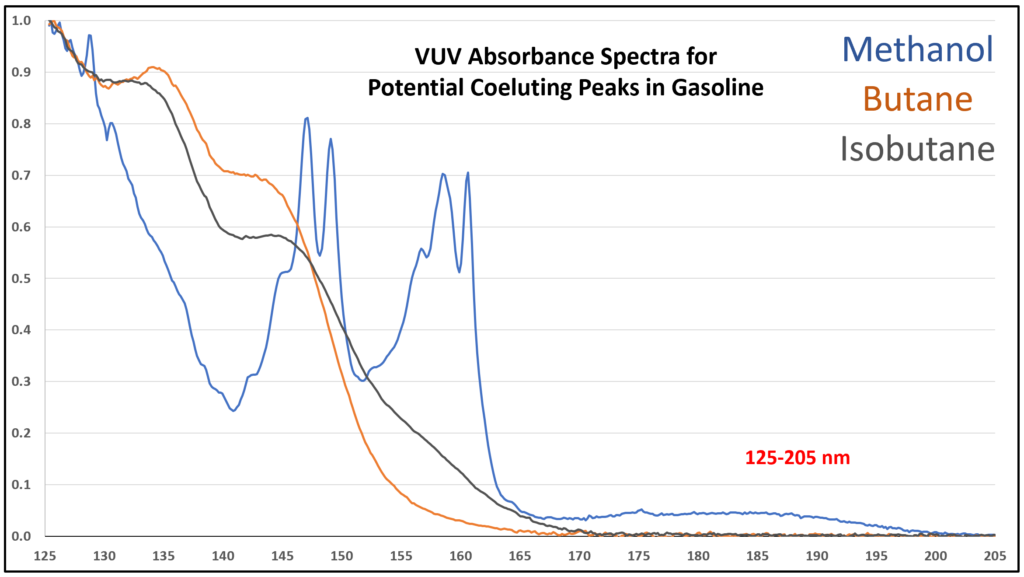
Figure 6. Zoom-in of VUV absorbance spectra (125-205 nm) for compounds that can coelute in gasoline analysis using GC-VUV allows close inspection of the absorbance features that enhance their deconvolution.








Leave a Reply