Published Jack Cochran on June 25, 2018
Characterization of Natural Gas Sweetening BTEX Hydrocarbon Contaminants by GC-VUV
Diglycolamine (DGA), also known as 2-(2-Aminoethoxy)ethanol, is used to “sweeten” natural gas by absorbing hydrogen sulfide (H2S) and carbon dioxide (CO2), so-called “acid gases” that lead to corrosion and other problems in gas delivery and processing systems. Typically, the DGA is mixed at a high percent concentration in water (H2O) and flowed in a countercurrent way to the natural gas to effect sweetening. In addition to taking out H2S and CO2, the DGA:H2O also picks up “heavy hydrocarbons”, a bit of an unusual moniker to me given they are actually volatile compounds like benzene, toluene, ethylbenzene, and xylenes (BTEX). Their removal from the DGA during its use or recycling is important to prevent operational problems and environmental pollution. While there has been much modeling, design, and operation effort to minimize BTEX accumulation in DGA in a process environment, there may still be a need to determine BTEX in DGA:H20 directly. That can be done with GC-VUV using the same setup shown previously for analyzing water and BTEX in triethylene glycol (TEG) used to dehydrate natural gas.
Figure 1 shows an example chromatogram from the GC-VUV analysis of BTEX and other hydrocarbons in 50:50 DGA:H2O. The GC run time was 13 min. Like the TEG analyzed previously, the DGA is extremely viscous, so samples were diluted 50:50 with ethanol for more reliable autosampler syringe fill. A 4mm Precision split liner with wool was used in the GC inlet. The GC column conditions are listed in Figure 1. The transfer line and flow cell of the VUV detector (VGA-100) were at 300°C, and nitrogen makeup gas was at 0.25 psi. Overlaid chromatograms in Figure 2 demonstrate the expected linear decrease in absorbance response as hydrocarbon concentrations in DGA:H2O go down.
Figure 3 zooms in on overlaid chromatograms for benzene (aromatic) and isooctane (isoparaffin) to show the excellent detectability afforded by GC-VUV for benzene, which is likely the most important “heavy hydrocarbon” to monitor in this application because of its environmental regulation. Isooctane shows a lower response, with an estimated detection limit between 10 and 20 ppm when using the full 125-240 nm VUV absorbance range. However, detectability can be improved by narrowing the plotted (and processed) absorbance range, something called “spectral filters” (Figure 4).
I end this blog post with another “first man on the moon” photo, the VUV absorbance spectrum for diglycolamine (Figure 5), which to my knowledge has never been seen before I pulled it off my own instrument in the lab. That spectrum is not only cool, but useful, as I will demonstrate in future writings.
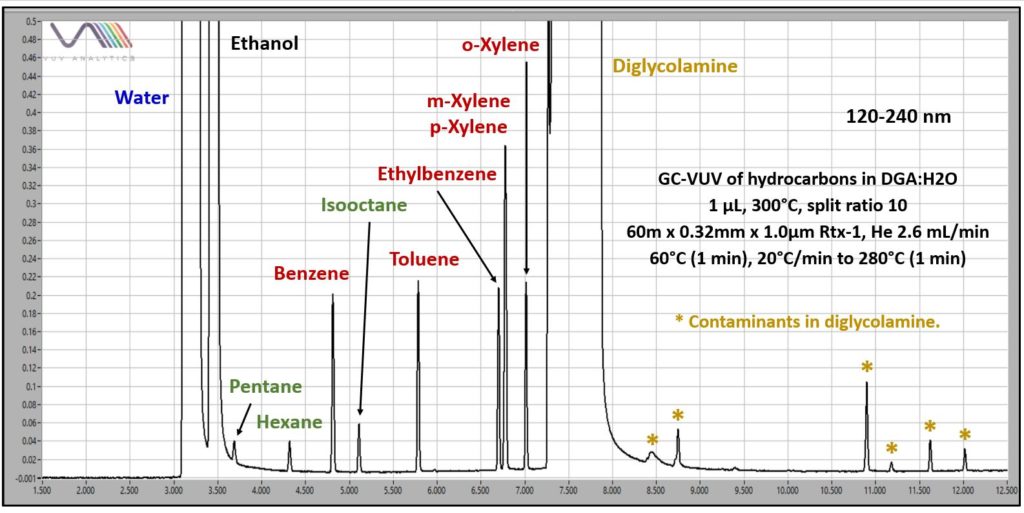
Figure 1. GC-VUV chromatogram of volatile hydrocarbons in a 50:50 DGA:H2O. Ethanol was used to dilute the DGA before GC analysis to allow for better autosampler operation.
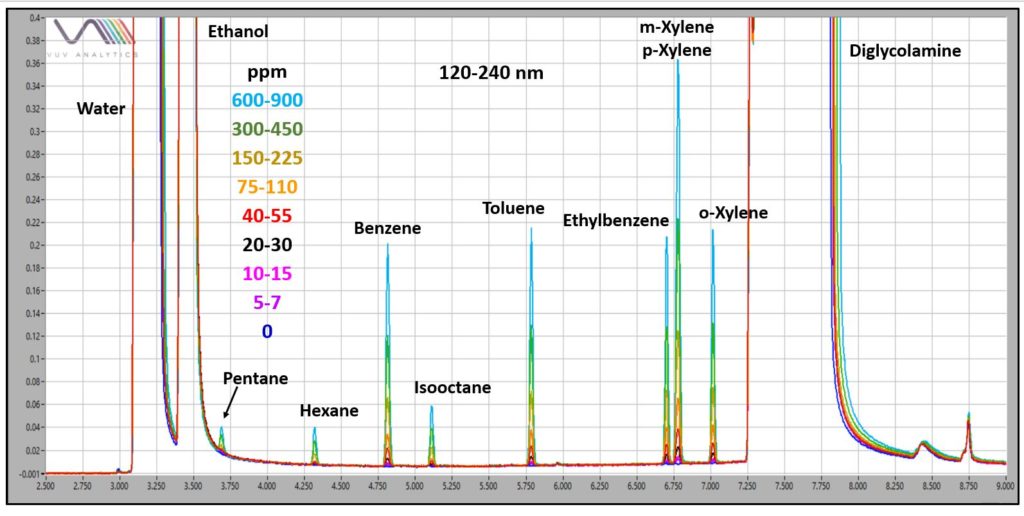
Figure 2. Overlaid GC-VUV chromatograms of volatile hydrocarbons in a 50:50 DGA:H2O. Detection can be accomplished in the low ppm (ng/µL) range for BTEX.
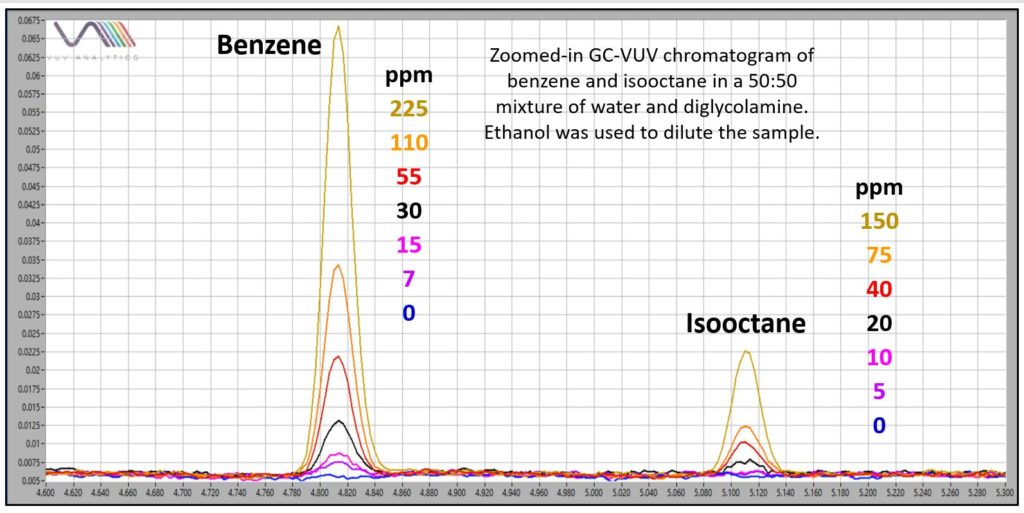
Figure 3. Overlaid GC-VUV chromatograms of volatile hydrocarbons in a 50:50 DGA:H2O showing good detectability for benzene. Isooctane has a lower VUV response.
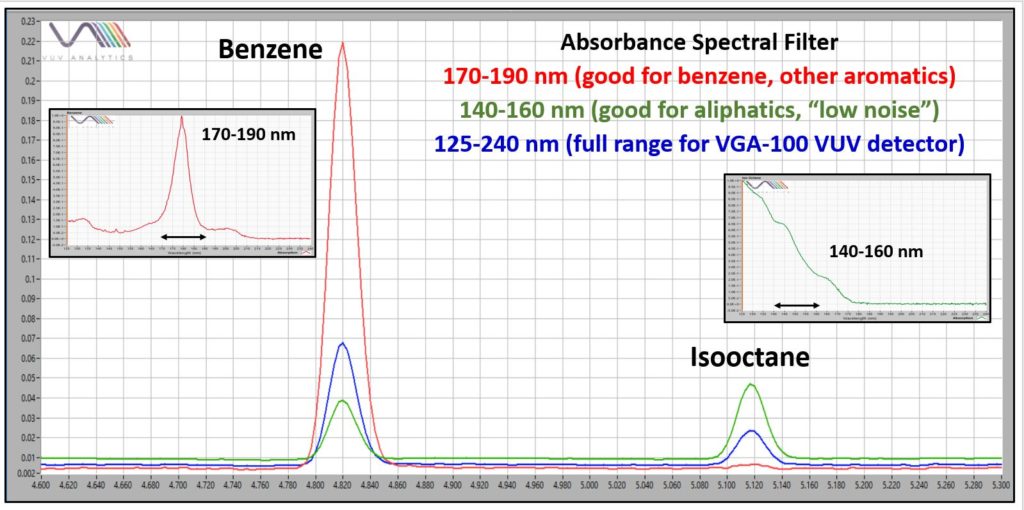
Figure 4. The use of appropriate spectral filters can improve limits of detection for both aromatic and aliphatic hydrocarbons.
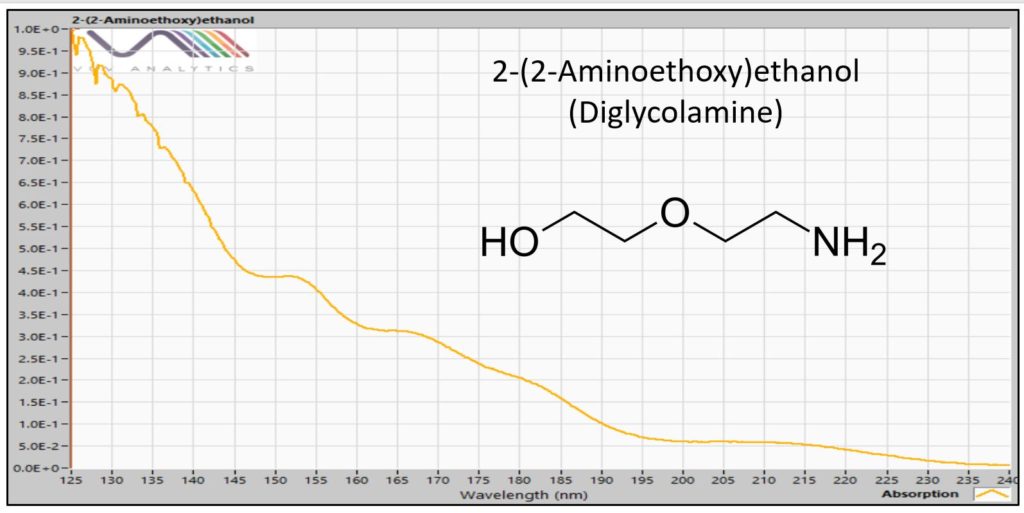
Figure 5. VUV absorbance spectrum for 2-(2-Aminoethoxy)ethanol (diglycolamine, DGA). The VGA-100 GC detector operates in the range of 125 to 240 nm.








Hi Jack:
would it be possible to see CO2 and H2S in this solution by VUV. They are likely under the water peak?
Also any idea on how low we could measure CO2 and H2S in an acid gas mixture (propane, methane ethane)?
Hi Chris,
If CO2 and H2S were eluting under a 25-50% water peak, they would almost certainly be invisible, as that much water would saturate the detector to at least 180 nm.
However, if we were to achieve well-separated peaks (at least from the large constituent peaks) for CO2 and H2S, we could quantitate down to about 1-5 ug on column (based on previous work done with CO2, CO, and CH4 in transformer oil).