Published Alex Hodgson on February 18, 2019
Part 3.5 – Snapshot of a Flow Cell Reaction
All you I Spy aficionados may have noticed something peculiar in one of the CDO-PTAD spectra in Part 3 namely 1,3-cyclohexadiene-PTAD. Its spectrum has several spectral “fingers” that the other spectra do not. Why is that? Let’s put on our organic chemistry caps and dig out those notes on reaction mechanisms. (DISCLAIMER: We cannot be held responsible for any traumatic flashbacks to your college days. We all had that one scarring class.)
Doing a quick perusal through the VUV spectra library, we find the “fingers” of our adduct spectrum line up perfectly with the “fingers” of carbon monoxide’s spectrum (Figure 1). This means that unless somehow the intact 1,3-cyclohexadiene-PTAD adduct happens to have this exact same feature, we’re seeing a “coelution” of the adduct (in some form) with carbon monoxide.
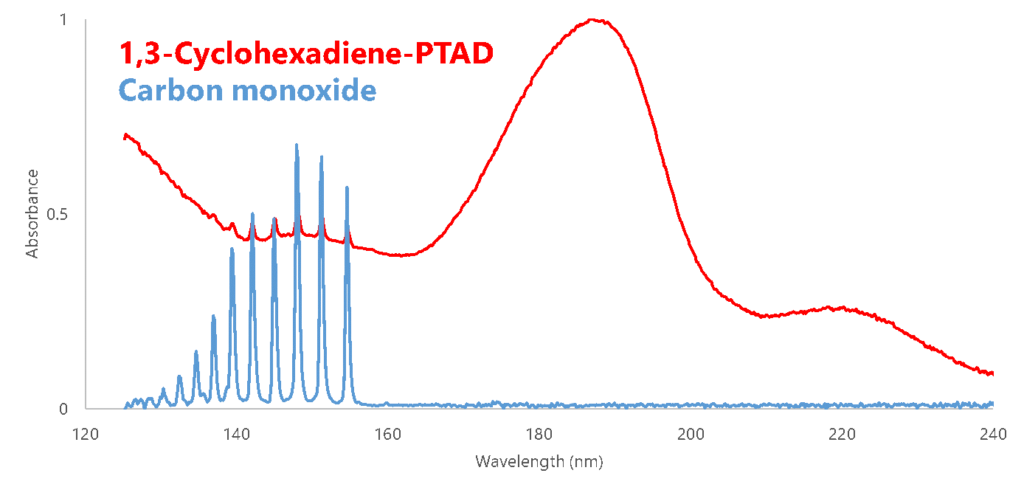
Figure 1. Overlaying the VUV spectra for 1,3-cyclohexadiene-PTAD and carbon monoxide, it looks to be a perfect match for the spectral “fingers” for each compound.
So where is that CO coming from? If we look at the structure of the CDO-PTAD adduct in Figure 2, we can envision a reaction mechanism by which we kick out a CO molecule; what the resulting structure would be is difficult to tell. Running the same sample on a GC-MS, the mass spectrum we get for 1,3-cyclohexadiene-PTAD shows molecular ion at 255 Da and an intense fragment ion at 227 that represents the loss of CO (Figure 3).
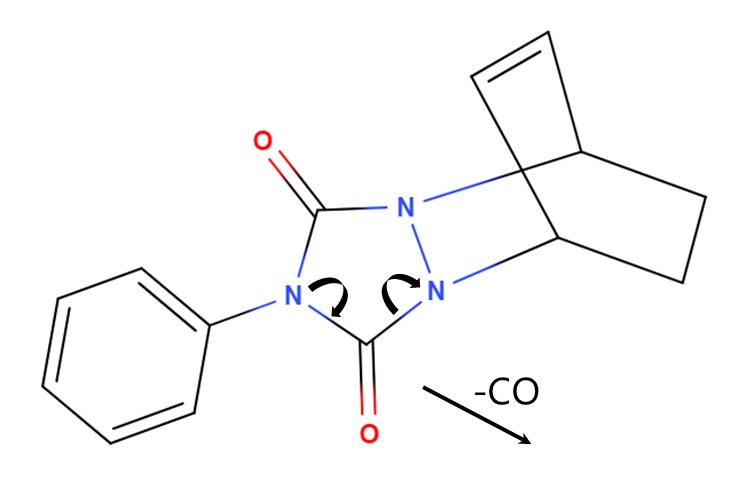
Figure 2. A potential mechanism for a flow cell/ion source reaction that would explain the loss of CO in both the VUV and mass spectra.
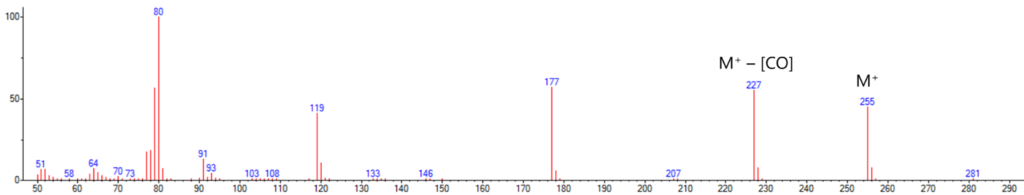
Figure 3. The mass spectrum of the same 1,3-cyclohexadiene-PTAD peak confirms a loss of CO in the detector itself, as we see response for both M+ and M+ – [CO] ions.








Leave a Reply