Published Dan Wispinski on June 9, 2020
In part two of this blog series I want to focus on the future of detailed hydrocarbon analysis. If you remember my first blog, I talked a little about my experience in shaping what DHA has become today. For me personally, what goes around comes around! All this time later I find myself once again helping to shape the future. That future uses vacuum ultraviolet spectroscopy (VUV) in an application called Verified Hydrocarbon Analysis™ (VHA) that maintains the advantages of DHA by GC-FID all while eliminating the drawbacks, resulting in more accurate data, increased productivity, and ease-of-use.
What is Vacuum Ultraviolet Spectroscopy and VHA you ask? Simply put VUV Spectroscopy takes advantage of the VUV region of the electromagnetic spectrum which is characterized by high-energy and short wavelengths of light from 125nm to 240nm. This region is special because every compound absorbs with the exception of carrier gasses like hydrogen, helium, and argon. Perhaps more importantly, every compound has a unique spectral fingerprint. These unique fingerprints make it possible to accurately identify or “verify” compounds of interest using a library of hydrocarbon spectra. The VHA library of spectra covers the vast majority of hydrocarbon compounds of interest.
One of the biggest challenges associated with the set up and optimization of the traditional 100-meter DHA method is achieving the separation necessary to ensure accurate identification of all compounds of interest – especially critical pairs. Critical pairs (figure 1) are closely eluting compounds of very similar boiling points that will often co-elute. To optimize this separation, a tuning column is often required and run times can be in excess of 140 minutes. Since VHA uses spectral fingerprints, critical pairs like benzene/1-methylcyclopentene and toluene/2,3,3-trimethylpentane need not be completely separated. As a result, shorter columns can be used which accelerates analysis time while ensuring accuracy.
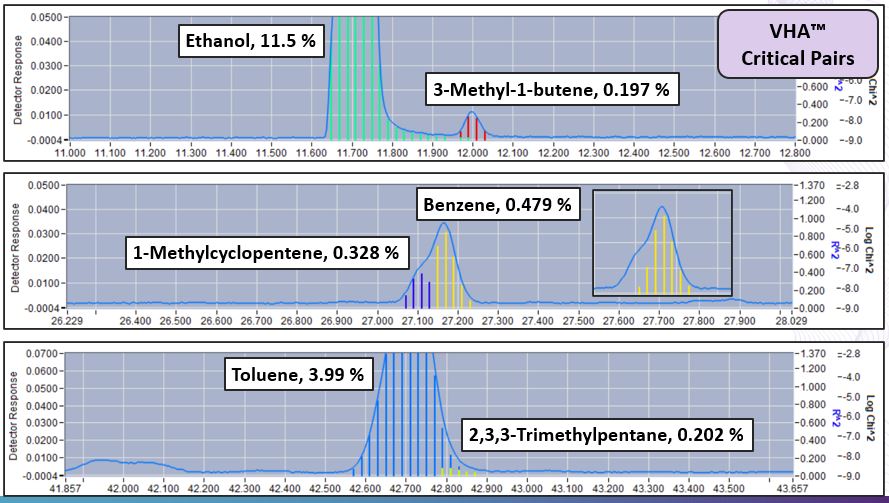
Figure 1. Critical Pairs
Misidentified peaks or peaks identified as unknown using DHA occur because even though hundreds of compounds are separated, identification depends solely on retention time and operator interpretation. Many coelutions still occur, often between hydrocarbon classes. VHA on the other hand identifies compounds and classes not only by a retention window but also by a compound’s unique spectral profile. The absorbance or response is directly proportional to concentration and allows accurate quantitation using similar normalization calculations as DHA. Just as DHA uses theoretical response factors, VHA uses the predictable response of analytes to enable a calibration free technique. During VHA data processing, the vast majority of gasoline compounds are “verified” by their unique spectra in the VHA library. In cases where an exact match is not made, the hydrocarbon class and carbon number is assigned. This is possible because compounds in a hydrocarbon class share spectral similarities. Since every time interval is analyzed, this results in zero “unknowns” compared to traditional DHA.
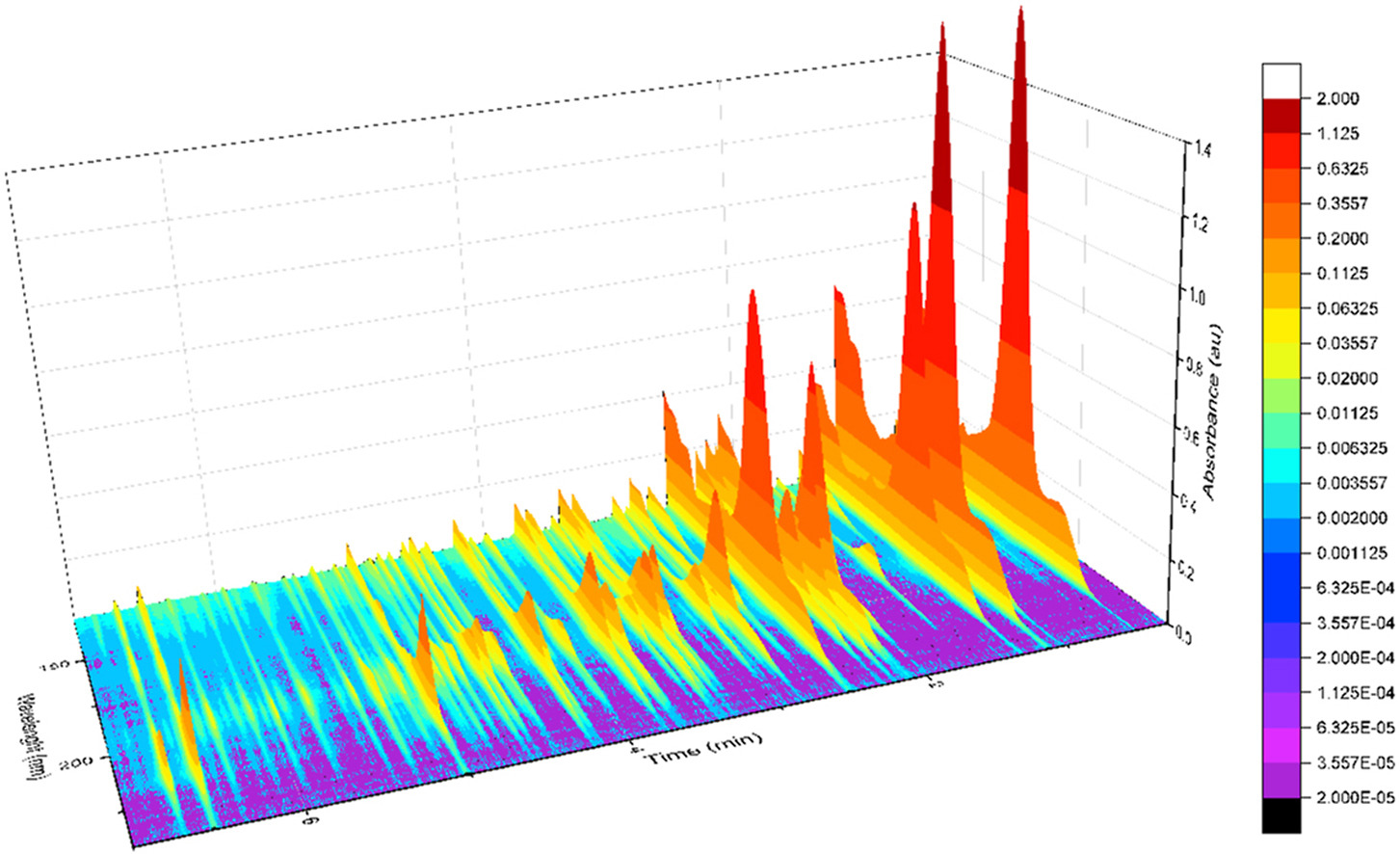
One of the core concepts of Vacuum Ultraviolet Spectroscopy is that traditional peak integration is not performed. There are no peak starts, peak ends, tangent drops or baseline forward/backward considerations that DHA analysts spend significant resources on. Even experienced operators may miss that the baseline follows valley to valley peaks points, omitting a large portion of FID response under a region of closely eluting compounds. This is a common DHA error source, especially on naphtha samples. Peak tailing (e.g. ethanol) or fronting can also cause problems for DHA. Instead, VUV spectroscopy uses Time Interval Deconvolution™ (figure 2) or TID™. These time intervals, similar to time slices in GC distillation methods, are discretely and sequentially analyzed to solve the entire chromatogram. This eliminates peak integration and allows automated processing.
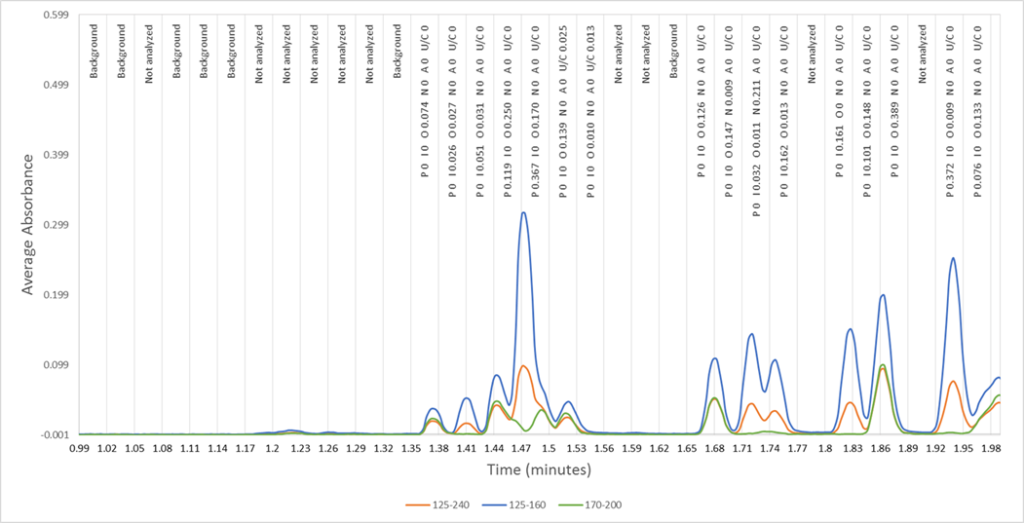
Figure 2. An example of Time Interval Deconvolution(TM) by VUV Analyze(TM) Software.
The future of hydrocarbon analysis is Verified Hydrocarbon Analysis. VHA provides the detailed analysis that is required while improving accuracy through spectral verification, increasing productivity due to faster analysis time and less human intervention and is easier-to-use because it is automated. This new ASTM method is currently on ballot.
In part three of this series I will take a deeper dive into VHA and discuss how it can be used across several different hydrocarbon streams.








A question : as I understand, VHA will, in a near
future, replace ASTM D6730, by idetification of
all individual hidrocarbon in naphtas and gasolines.
Am I right ?
What is probable time to be ready ?
Hi Raul — Thank you for your comment! Here is the feedback from Dan Wispinski, the blog author:
ASTM D6730 can not identify all individual hydrocarbons because 1.) up to 5% in gasoline and sometimes >5% in naphtha are labelled as unknown
2.) identified hydrocarbons in D6730 are often mis-labelled and
3.) D6730 can not deal with coelutions and labels a hydrocarbon even though there may be several compounds in the peak
VHA verifies 85 to 100% of individual compounds in gasoline and naphtha without peak integration and operator interpretation. In up to 15% of the cases where an exact library match to an individual hydrocarbon can not be made then the hydrocarbon is assigned a carbon number based on retention index and a hydrocarbon class based on spectral response. This results in zero% unknowns which is a significant benefit over misidentifications or coelutions .
VHA is available now. The ASTM method based on VHA is in ballot process and will be available in 6 to 12 weeks.
Let us know if you have any further questions!
Hello Dan & Sean,
Nice to see how Ethanol tails !
We should calculate VHAtoD86 and DHAtoD86 and compare D86 data ! Nice publication for JoHiResChro for sure !